Jul 30 2013
Hydrogen is deceptively simple. It has only a single electron per atom, but it powers the sun and forms the majority of the observed universe. As such, it is naturally exposed to the entire range of pressures and temperatures available in the whole cosmos. But researchers are still struggling to understand even basic aspects of its various forms under high-pressure conditions.
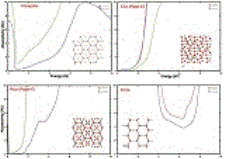 The predicted optical absorption of a 1 ìm of hydrogen in a high pressure diamond anvil cell for different crystal structures at a pressure of 300 GPa (3 million times normal atmosphere—similar to the pressure in the center of the Earth). At these pressures hydrogen no longer forms molecules, but instead forms in sheets, as shown in the figure. Graph is courtesy of Ronald Cohen.
The predicted optical absorption of a 1 ìm of hydrogen in a high pressure diamond anvil cell for different crystal structures at a pressure of 300 GPa (3 million times normal atmosphere—similar to the pressure in the center of the Earth). At these pressures hydrogen no longer forms molecules, but instead forms in sheets, as shown in the figure. Graph is courtesy of Ronald Cohen.
Experimental difficulties contribute to the lack of knowledge about hydrogen’s forms. The containment of hydrogen at high pressures and the competition between its many similar structures both play a part in the relative lack of knowledge.
At high pressures, hydrogen is predicted to transform to a metal, which means it conducts electricity. One of the prime goals of high pressure research, going back to the 1930s, has been to achieve a metallic state in hydrogen. There have been recent claims of hydrogen becoming metallic at room temperature, but they are controversial.
New work from a team at Carnegie’s Geophysical Laboratory makes significant additions to our understanding of this vital element’s high-pressure behavior. Their work is published in two papers by Proceedings of the National Academy of Sciences and Physical Review B.
New theoretical calculations from Carnegie’s Ronald Cohen, Ivan Naumov and Russell Hemley indicate that under high pressure, hydrogen takes on a series of structures of layered honeycomb-like lattices, similar to graphite. According to their predictions the layers, which are like the carbon sheets that form graphene, make a very poor, transparent metal. As a result, its signature is difficult to detect.
“The difficulty of detection means that the line between metal and non-metal in hydrogen is probably blurrier than we’d previously supposed,” Cohen said “Our results will help experimental scientists test for metallic hydrogen using advanced techniques involving the reflectivity of light.”