Feb 11 2016
Tiny rocketlike particles that move around on their own in a hydrogen peroxide solution can detect trace amounts of the lethal toxin ricin within minutes (ACS Sens. 2016, DOI: 10.1021/acssensors.5b00300). They could someday provide a quick, easy way to detect the bioterrorism agent in food and water samples without having to bring them back to a lab. The particles, made of graphene oxide and platinum, carry sensor molecules that glow when they bind to ricin.
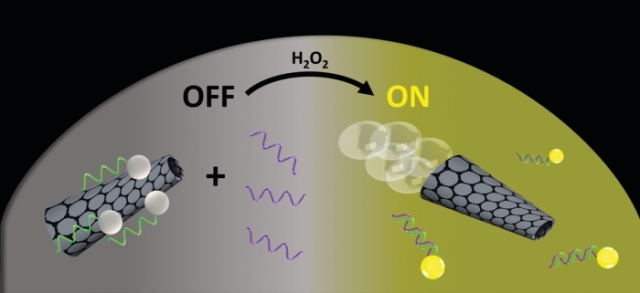 A new ricin-detection method uses micromotors (gray cones) modified with a DNA aptamer (green) attached to a fluorescent dye (white spheres). While the dye-aptamer complexes are attached to the micromotors (left), the dye’s glow is quenched (OFF). As the micromotors propel themselves around (right), using hydrogen peroxide (H2O2) as fuel, they encounter ricin (purple). The dye-aptamer complexes bind to the toxin, releasing from the micromotors and recovering their fluorescence (ON).Credit: ACS Sens.
A new ricin-detection method uses micromotors (gray cones) modified with a DNA aptamer (green) attached to a fluorescent dye (white spheres). While the dye-aptamer complexes are attached to the micromotors (left), the dye’s glow is quenched (OFF). As the micromotors propel themselves around (right), using hydrogen peroxide (H2O2) as fuel, they encounter ricin (purple). The dye-aptamer complexes bind to the toxin, releasing from the micromotors and recovering their fluorescence (ON).Credit: ACS Sens.
Current methods to detect the protein ricin rely on chromatography and spectroscopy, which take hours. Researchers have recently developed sensitive ricin biosensors based on aptamers: short DNA sequences with strong affinity for specific protein targets. These biosensors show a slow response because of the time it takes for them to bind to the target ricin.
Joseph Wang and his team of nanoengineers at the University of California, San Diego, wanted to make aptamer sensors faster and simpler to use. So they took advantage of self-propelling chemical motors developed in their laboratory and attached ricin-specific apatamers tagged with fluorescent dyes. The idea was that the swimming motors could actively seek out ricin in a sample and speed up detection.
The motors are 10-µm-long, 5-µm-wide tubes made of graphene oxide lined with platinum. In a dilute hydrogen peroxide solution, the platinum catalyzes the breakdown of the peroxide into water and oxygen. The oxygen bubbles shoot out one end of the tube, propelling them in the liquid like little rockets.
The dye-aptamer complexes are attached noncovalently to the motors via interactions between the pi electrons in graphene and the aptamer, which quench the dye’s glow. But when the motors come close to ricin, the aptamer releases graphene to latch onto the protein, allowing the dye to glow.
To test the sensors, the researchers added micromotors to ricin solutions with concentrations varying from 0.1 ng/mL to 10 µg/mL and took fluorescence images after three minutes. Increasing ricin concentration increased the fluorescence intensity. Some state-of-the-art lab-based methods can detect ricin concentrations lower than 0.1 ng/mL, but they are much slower. Ricin can be lethal at a dose of a few tens of ng/mL.
Then, to test the effect of different motor speeds, they added droplets containing different concentrations of hydrogen peroxide fuel to a 10-µg/mL ricin sample. Solutions with no fuel showed a dim glow, recovering around 12% of the quenched fluorescence, while those with 2% fuel concentration recovered their full fluorescence. “The micromotor movement accelerates the contact between sample and receptor and hence the detection process,” Wang says.
The researchers also tested the sensors in tap water, saliva, and urine samples spiked with 10-µg/mL ricin and 1% hydrogen peroxide. All samples recovered at least 65% of their original brightness within three minutes.
Martin Pumera, a chemist at Nanyang Technological University, calls the technique “highly innovative.” It has “unprecedented speed when compared to other analytical techniques,” he says.
“This is a first step towards an efficient, simple, on/off visual test for the presence of ricin,” says Andrew J. Bonham, a chemist at Metropolitan State University of Denver. “The robust response of the aptamer-micromotor biosensor in complex media, including saliva and urine, is very promising.” The technique could be expanded to a variety of biological threats using different aptamers, he says, but right now detection requires an optical microscope with fluorescence filters. For field use, they would need a more portable fluorescence imaging system.
Wang says that his team plans to address that challenge. They are working with colleagues to develop a fluorescent microscope device that can attach to a smartphone camera.