Mar 25 2016
Penn State researchers have developed an innovative technique to rotate cells, individual particles, or organisms by utilizing acoustic waves in a microfluidic device. This latest advancement will enable scientists to capture 3D images, simply using a cell phone.
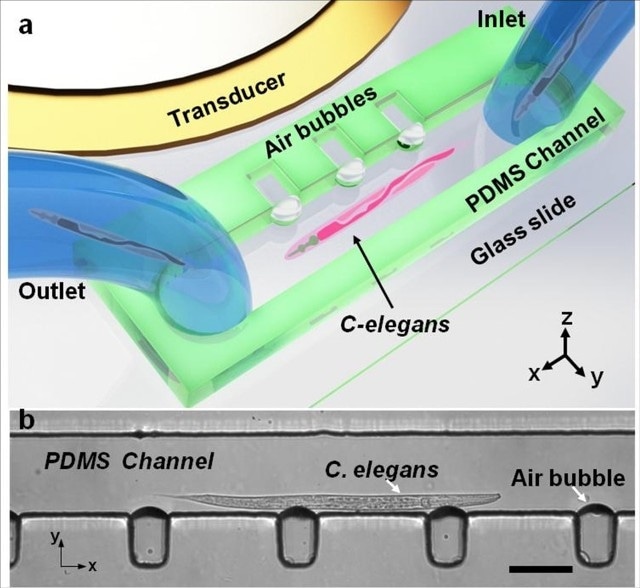 Design and operation of the acoustofluidic rotational manipulation (ARM) device. (a) A schematic of the experimental setup. The piezoelectric transducer that generates acoustic waves is placed adjacent to the microfluidic channel. The acoustic waves actuate air microbubbles trapped within sidewall microcavities. (b) An optical image showing a mid-L4 stage C. elegans trapped by multiple oscillating microbubbles. Scale bar = 100ìm. Image: Tony Jun Huang/Penn State
Design and operation of the acoustofluidic rotational manipulation (ARM) device. (a) A schematic of the experimental setup. The piezoelectric transducer that generates acoustic waves is placed adjacent to the microfluidic channel. The acoustic waves actuate air microbubbles trapped within sidewall microcavities. (b) An optical image showing a mid-L4 stage C. elegans trapped by multiple oscillating microbubbles. Scale bar = 100ìm. Image: Tony Jun Huang/Penn State
Generally, acoustic waves can shift and place biological samples along the three axes: x, y and z. The Penn State team has now used these acoustic waves to safely and gently rotate the biological specimens. This marks a major breakthrough in the field of single-cell analysis, organism, and drug discovery research.
The study, headed by Tony Jun Huang, professor of engineering science and mechanics and Huck Distinguished Chair in Bioengineering Science, has been reported in the Nature Communications journal.
An acoustofluidic rotational manipulation (ARM) technique, developed by Huang and his research team, captures bubbles in a set of tiny cavities within a microfluidic device. Analogue to ultrasound imaging transducers, acoustic transducers produce an acoustic wave in the fluid, causing the bubbles to vibrate and this in turn produces microvortexes in the flowing liquid that can be easily adjusted. As a result, the sample can rotate at any required speed and in any direction.
Currently confocal microscopes are required in many biological, biochemical and biomedical studies, but many labs do not have access to a confocal microscope, which costs more than $200,000. Our ARM method is a very inexpensive platform and it is compatible with all the optical characterization tools. You can literally use a cell phone to do three-dimensional imaging.
Tony Jun Huang, Professor of Engineering Science and Mechanics, Penn State
To show the capabilities of the microfluidic device in a better way, the researchers worked with a model organism, C. elegans, which measures 1 mm in length and is often employed in biological studies. This model organism was acoustically rotated, including a HeLa cancer cell that was also acoustically rotated and imaged.
In current techniques, manipulation of tiny objects mostly relies on the specimen’s electrical, magnetic or optical properties, and laser heating may also cause damage to the sample. In contrast, the ARM technique employs a gentle acoustic wave that is produced by a power analogous to ultrasound imaging; this acoustic wave is generated at reduced frequency. In addition, the device is small and can be used easily.
Our method is a valuable platform for imaging and studying the effect of rotation at the single cell level. More important, with the capacity to rotate large numbers of cells in parallel, researchers will be able to perform high-throughput single-cell studies.
Adem Ozceki, Graduate Student, Engineering Science and Mechanics, Penn State
Thus, ARM technology could be applied to a variety of physical and biological science analysis. In addition to this, it showed good biocompatibility in a HeLa cell viability test, where 99.2% of cells remained unaffected after manipulation.
Other researchers who contributed to the study were former group member Daniel Ahmed, Ph.D.; Wendy Hanna-Rose, associate professor of biochemistry and molecular biology; and graduate students Nitesh Nama, Nagagireesh Bojanala, Yuchao Chen, and Awani Upadhyay, all from Penn State. The study is titled, "Rotational manipulation of single cells and organisms using acoustic waves". The research was carried out at the Penn State Materials Research Institute's Nanofabrication Laboratory.
The study was supported by the National Institutes of Health; the Center for Nanoscale Science, an NSF Materials Research Science and Engineering Center at Penn State; and National Science Foundation.