Jul 14 2016
A research team of Satoshi Ishii, MANA scientist, and Tadaaki Nagao, group leader, Nano-System Photonics Group, International Center for Materials Nanoarchitectonics (MANA), NIMS, discovered through numerical calculations that nanoparticles of transition metal nitrides and carbides absorb sunlight very efficiently, and confirmed experimentally that nitride nanoparticles, when dispersed in water, quickly raise water temperature. These nanoparticles may be applied for heating and distillation of water through efficient sunlight use.
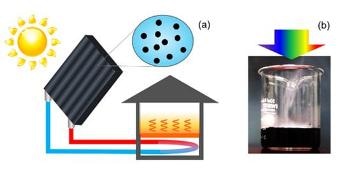 Diagram showing a solar water heating system using nanoparticles. (b) Application of condensed light from a solar simulator to water with dispersed TiN nanoparticles. Note that rising water vapor can be seen even before the water temperature increases.
Diagram showing a solar water heating system using nanoparticles. (b) Application of condensed light from a solar simulator to water with dispersed TiN nanoparticles. Note that rising water vapor can be seen even before the water temperature increases.
Sunlight is one of the most promising renewable energies. The examples of sunlight use are power generation using solar cells and water heating through photothermal conversion, a process in which absorbed sunlight is converted into heat. Water and air heating accounts for 55% of household energy consumption. If sunlight can be converted into heat very efficiently, it is possible to heat water and air without using electricity, leading to reduction of carbon dioxide emissions. Absorption of sunlight using conventional solar heat collector panels and heat collector tubes results in loss of heat through conduction. For this reason, nanoparticles that can directly heat media including water when they are dispersed in the media are attracting attention.
Recently, the research team and Naoto Umezawa, senior researcher, Catalytic Materials Group, Environmental Remediation Materials Unit, Environment and Energy Materials Division, NIMS, jointly performed first-principles calculations to search for nanoparticle materials suitable for photothermal conversion and to estimate their physical properties. As a result, the team found that transition metal nitrides and carbides, which are ceramics, very efficiently absorb sunlight. Furthermore, after choosing titanium nitride (TiN) out of a number of transition metal nitrides, the team dispersed TiN nanoparticles in water, and applied sunlight to the aqueous solution. In this experiment, the team confirmed that the nanoparticles converted sunlight into heat at high efficiency of nearly 90%. Since TiN nanoparticles exhibit broadband plasmon resonances, their sunlight absorption efficiency is likely to be higher than those of gold and carbon nanoparticles on a per nanoparticle basis. In future studies, the team is planning to apply these results to floor heating, water heating, and distillation of sewage and seawater. Besides these projects, the team is also working on other applications of nanoparticles such as the development of hybrid materials between polymers and nanoparticles and the study of nanoparticle-mediated chemical reactions.
This research was conducted as a part of the study "Interface electromagnetic field control and use of heat energy in the ceramic hetero-layer" (principal investigator: Tadaaki Nagao) in the research area "Phase interface science for high-efficient energy use" (research supervisor: Katsunori Hanamura) supported by JST’s Strategic Basic Research Programs (CREST).
The part of this study related to numerical calculations and experiments was published in the Journal of Physical Chemistry C on January 25, 2016.