Nov 18 2016
 A spinning “double helix” phase mask turns the single-point image of a molecule into barbell-shaped lobes that change angle depending on the time the image is captured. A molecule may be captured multiple times in a single image. Credit: Landes Research Group.
A spinning “double helix” phase mask turns the single-point image of a molecule into barbell-shaped lobes that change angle depending on the time the image is captured. A molecule may be captured multiple times in a single image. Credit: Landes Research Group.
Researchers at Rice University have developed a new technique, known as super temporal resolution microscopy (STReM), to capture images of chemical processes that occur relatively faster than the ability of most laboratory cameras to grab them.
The technique enables the researchers to observe and collect significant information related to fluorescing molecules at a frame rate that is 20 times faster than conventional lab cameras.
The research carried out by Rice chemist Christy Landes and her colleagues, in collaboration with Rice electrical engineer Kevin Kelly, has been published in the Journal of Physical Chemistry Letters of the American Chemical Society (ACS).
Initially, the Rice researchers employed a Nobel-winning microscopy procedure that observes objects such as molecules at “super resolution”, i.e. particles inferior to the diffraction limit and very minute for most microscopes to observe.
Super-resolution microscopy lets us image things smaller than about half of visible light’s wavelength - around 250 nanometers.
Christy Landes, Chemist, Rice University
But she noted a barrier: “You couldn’t take pictures of anything faster than your frame rate,” she said.
The Rice lab's recent enhancement, which uses a rotating phase mask to encode high-speed dynamics in each camera frame, will enable the researchers to perceive the processes happening at interfaces such as desorption and adsorption of proteins or molecules' trajectories when they travel along 2D surfaces.
According to Landes, conventional charge-coupled device (CCD) cameras have maximum frame rates of 10 - 100 ms. Although other techniques such as electron microscopy enable materials to be viewed at subnanoscale, super-resolution microscopy can be applied for fragile samples such as biomolecules. This is because the biomolecules are not destroyed in the process.
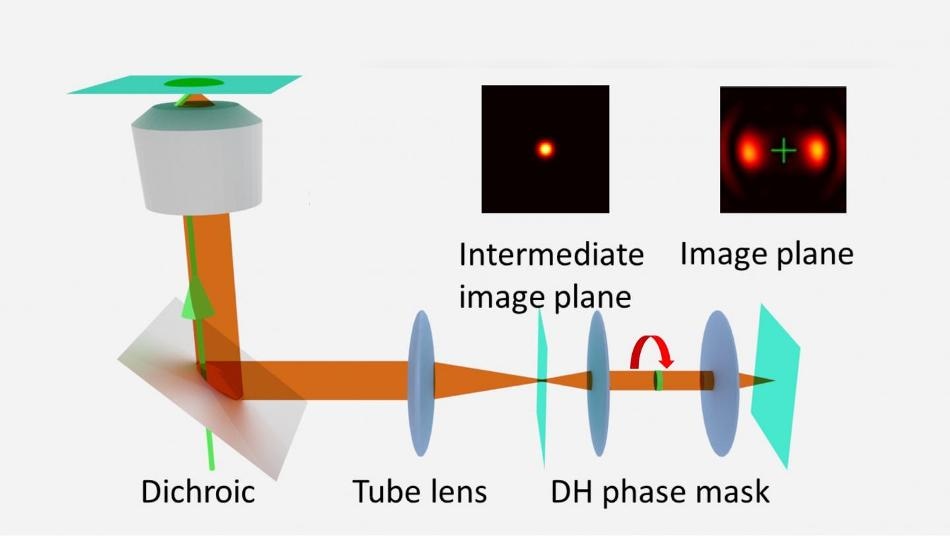
The technique manipulates the phase of light to provide a highly complicated shape to the image at the detector. Earlier the technique was employed by other researchers to encode the position of an object in a 3D space within an otherwise 2D image.
The Rice lab’s contribution was to note that by manipulating the phase over time, it would also be possible to encode faster time resolutions within a slow image frame. So, the team designed and developed a spinning phase mask.
The resulting images capture dynamic events occurring faster than the intrinsic frame rate of the camera. The shape of each of the images in a frame effectively provides a unique time stamp to the image.
The technique exploits the properties of microscopy well known to people who have, at some point, captured a blurry picture. Point spread functions describe the shape of images that are in focus as well as out of focus. For particles as small as single molecules, moving in and out of focus occurs easily, and the shape and size of the resultant blur enable the researchers to perceive the positioning of the particle from the focal plane.
Phase-mask engineering enables focus-dependent blur for detection to be carried out in a simple manner by introducing discrete point spread functions. When viewed on a film, they resemble the lobes of a barbell and rotate with respect to the focus.
According to Landes, STReM uses the changes in point spread function of the spinning mask to gather temporal information. The new technique enables tracking of the time point at which an event takes place within each frame by observing the changes in the angles of the lobes.
The purpose is to allow scientists to study fast processes without the need to buy faster and much more expensive cameras. This involves extracting more information from single images.
Wenxiao Wang, Graduate Student, Rice University
Landes - who recently won the prestigious Early Career Award in Experimental Physical Chemistry awarded by ACS for her research toward integrating super-resolution microscopy with information theory to perceive protein separations - stated that only a few hundred dollars were spent by the lab to design and develop the mechanism, which is far less than the cost of purchasing a faster camera.
The research work by Kelly formed the basis of the phase mask, where he employed Rice’s single-pixel camera to develop a piece of plastic with variable thickness and the ability to distort light traveling to the CCD.
Like the single-pixel camera, we’re doing compressive analysis. With the static phase mask, three-dimensional information is compressed into a 2-D image. In this particular case, we have compressed faster information into a slower camera frame rate. It’s a way to get more information in the pixels that you have.
Christy Landes, Chemist, Rice University
Postdoctoral research associates Hao Shen and Lawrence Tauzin Co-authors, and graduate students Bo Shuang, Benjamin Hoener, and Nicholas Moringo are the co-authors of this study. Kelly is an associate professor of electrical and computer engineering. Landes is an associate professor of electrical and computer engineering as well as chemistry.
The research was supported by the National Science Foundation and the Welch Foundation.
Rice University/Youtube.com