Jul 30 2018
In cancer treatment, chemotherapy is not a scalpel, but a cleaver. While chemotherapy effectively fights tumors by attacking quickly dividing cells, it also destroys healthy cells in the scalp, bone marrow, gut, and other organs, resulting in adverse side effects. In other words, while these toxic chemicals save lives, they do so by compromising the well-being of patients.
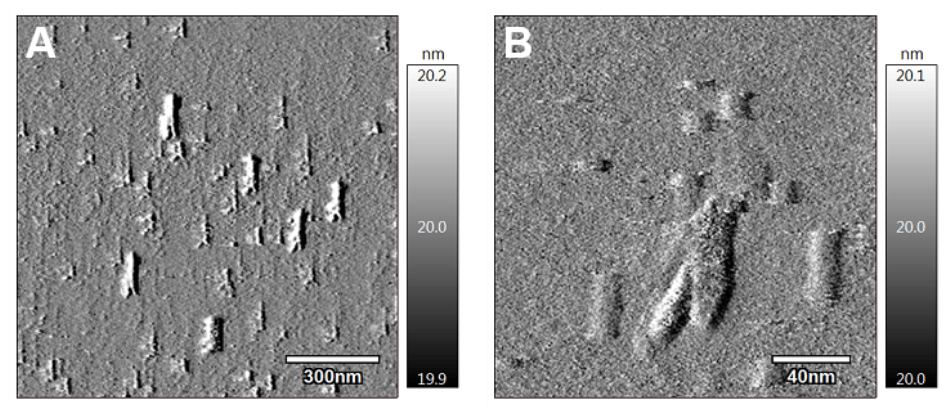 Atomic-scale images of gemcitabine-loaded nanoparticles reveal an unusual cylindrical shape. Courtesy of Tony Tam.
Atomic-scale images of gemcitabine-loaded nanoparticles reveal an unusual cylindrical shape. Courtesy of Tony Tam.
Glen Kwon, a professor in the University of Wisconsin–Madison School of Pharmacy, is investigating the use of nanoparticles that can improve the therapeutic properties of these drugs. Kwon undertook this study in an effort to tip the balance toward the benefits of chemotherapy.
Ina recent work that appeared in the journal ACS Nano, Kwon’s laboratory created a stabilized form of gemcitabine - a standard chemotherapy agent - enclosed in nanoparticles that can slow down their release. In mouse models simulating human lung cancer, the enhanced drug inhibited the growth of tumors more effectively when compared to the normal gemcitabine.
“There’s been a lot of hype about nanotechnology,” states Kwon, who has worked in the field for over two decades. “It’s an ambitious goal: to target drugs to particular places in our body.”
Kwon says that while that objective may be some way off, particles that carry drugs into the body - called nanocarriers - are already showing to be effective. “What nanocarriers can do is reduce toxicity,” says he.
Working alongside with his graduate student Tony Tam a couple of years ago, Kwon created an enhanced system for delivering paclitaxel - a chemotherapy drug usually marketed as Taxol—at the time of treatment. Tam fixed a short lactic acid chain to the drug, which allowed it load into nanocarriers made partly of lactic acid. The nanocarrier has previously been used in humans.
Therefore, when turning to the common chemotherapy agent, gemcitabine, Tam employed the same strategy - that is, adding on lactic acid chains and loading it into the nanocarrier.
“But the first trial that we had was not stable at all,” states Tam, currently a senior scientist at Merck in San Francisco. In order to boost the stability of the drug, he turned to three-decade-old work out of Japan.
In the 1980s, Kyoto University researchers integrated lactic acid chains that were equal except for one main characteristic - their handedness. A large number of molecules come in mirror-image forms of themselves, and when the Japanese team integrated right- and left-handed forms of lactic acid polymers, the ensuing crystals, known as stereocomplexes, were observed to be much more stable.
The stability of the drug spiked when Tam created gemcitabine-linked stereocomplexes of lactic acid and then loaded them into the nanocarrier. Compared to gemcitabine, which was attached solely to the left-handed version of lactic acid, the stereocomplex expelled gemcitabine 15 times more gradually in a synthetic solution.
As gemcitabine breaks down so rapidly in the body, it is often administered at high doses to ensure that it sufficiently reaches the tumor. In case this nanocarrier system slowed release in the body, it would mean gentler doses have to be given.
Peculiarly, the nanoparticles adopted an unpredicted shape. Although similar types of nanocarriers encase drugs in a sphere, images obtained at the atomic scale reveal the gemcitabine stereocomplexes that take a stretched-out, cylindrical shape. 10,000 of these cylinders could be stacked end-on-end within the thickness of a piece of paper.
In mice simulating a human-derived non-small-cell lung cancer line, treatment with the stereocomplexed nanocarriers stretched over three weeks inhibited the growth of tumors. However, in the mice treated with standard gemcitabine, the tumors more than doubled in size. The mice were treated at a relatively low dose in order to evaluate the variations between the versions of gemcitabine.
“Ultimately our goal is to get this into human beings,” says Kwon, adding that many steps, for example, scaling up preliminary safety studies and production, will be needed.
With the Wisconsin Alumni Research Foundation, Tam and Kwon submitted a patent based on their changes to gemcitabine that improved its release and stability.Kwon also co-founded a preclinical stage company called Co-D Therapeutics, which develops nanocarrier-based medicines based on his previous work with paclitaxel.
“This research relied on really good teamwork in the School of Pharmacy community,” states Tam, observing that members of two other laboratories in the school contributed to the ACS Nano paper.
“Collaboration is very important.”
This work was supported in part by the National Institutes of Health (grant R01AI01157) and the National Science Foundation (award CHE-9974839).