Aug 13 2019
Although hexagonal-boron nitride (h-BN) is known to be tough, researchers at Rice University are making this material easier to get along with.
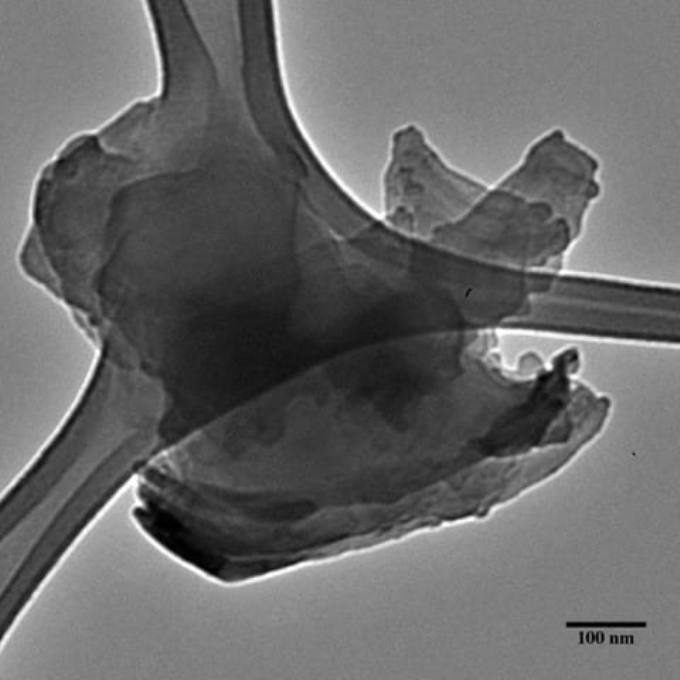 A flake of functionalized hexagonal-boron nitride created at Rice, as seen under a transmission electron microscope. (Image credit: The Angel Martí Group)
A flake of functionalized hexagonal-boron nitride created at Rice, as seen under a transmission electron microscope. (Image credit: The Angel Martí Group)
The two-dimensional (2D) h-BN is an insulating material and is also referred to as “white graphene.” It is an excellent conductor of heat—an advantage for composites that depend on it to improve their properties—and is also four times stiffer than steel. These qualities make it hard to modify h-BN.
In addition, its close hexagonal lattice of alternating nitrogen and boron atoms is extremely resistant to change, unlike other 2D materials, including graphene, that can be easily altered—aka functionalized—with other types of elements.
A protocol to improve h-BN with carbon chains has been published by the Rice lab chemist Angel Martí. This process changes the 2D hard h-BN into a material that is more conducive to bonding with polymers or other types of materials in composites but still retains its strength.
The laboratory’s paper, reported in the American Chemical Society’s Journal of Physical Chemistry, indicates that the h-BN material can also be made more dispersible in organic solvents.
Along with his team, Martí altered the Billups-Birch reaction process, which they had effectively utilized to modify boron nitride nanotubes in order to attack the defenses of covalently attached carbons and h-BN.
In 2004, Rice Professor Emeritus of Chemistry Edward Billups improved birch reduction to functionalize carbon nanotubes. Initially discovered in the 1940s, birch reduction frees electrons to adhere to other atoms. In the Rice protocol, Martí and his group were able to regulate the amount of h-BN functionalization by changing the amount of lithium in the reaction.
Since lithium is an alkali metal, it sheds free electrons when integrated with liquefied ammonia. When combined with a carbon source—1-Bromododecane in this case—and h-BN flakes, the reaction creates an alkyl radical, a type of chemical species that reacts with h-BN and forms a bond.
According to Martí, this is the best technique discovered so far to alter h-BN, which is resistant to change even under extreme temperatures.
You take a little bit of graphite and put it in a furnace at 800 degrees (Celsius), and it will be gone. You take hexagonal-boron nitride and do the same, and it will still be there smiling at you.
Angel Martí, Chemist and Associate Professor of Chemistry, Bioengineering, and Materials Science and Nanoengineering, Rice University
“That gives you an idea of how stable it is, and that’s the problem we wanted to address,” Martí stated. “The material is good for certain applications, but to control its properties for manufacturing, you have to graft different groups onto the surface.”
A 20-to-1 molar ratio of lithium to h-BN improved the process of grafting carbon chains to the edges and surface, he said. Since the base h-BN continues to be stable under extreme temperatures, it can be returned to its perfect state by merely burning off the functional chains.
H-BN is known to be naturally hydrophilic or water-attracting, but the functional carbons render them almost superhydrophobic or water-avoiding—an excellent trait for making protective films, Martí added. However, even when the flakes are improved, they remain conducive to dispersion in non-polar solvents.
Martí informed that his team is investigating other types of molecules that can possibly be grafted onto white graphene.
What about benzene groups? What about ethers? What about groups that will make it compatible with other materials? There’s a lot of interest in making composite materials between h-BN, boron nitride nanotubes and polymers. Ultimately, we’d like to graft different groups onto h-BN and build a library, kind of a toolbox, of functional groups that can be used with these materials.
Angel Martí, Chemist and Associate Professor of Chemistry, Bioengineering, and Materials Science and Nanoengineering, Rice University
The lead author of the paper is Rice alumnus Carlos de los Reyes. Co-authors are Rice undergraduate Katharyn Hernández, graduate students Cecilia Martínez-Jiménez, Cedric Ginestra, and Ashleigh Smith McWilliams, research assistant Kendahl Walz-Mitra and Matteo Pasquali, the A.J. Hartsook Professor of Chemical and Biomolecular Engineering, a professor of materials science and nanoengineering and of chemistry.
Martí is an associate professor of chemistry, of bioengineering and of materials science and nanoengineering.
The study was supported by the National Science Foundation, the Air Force Office of Scientific Research, and the Welch Foundation supported the research.