Scientists from the Tokyo Institute of Technology discovered that a unique hybrid ferritin nanocage with histidine residues exhibits 1.5 times higher metal ion uptake and enhanced catalytic efficiency for alcohol production.
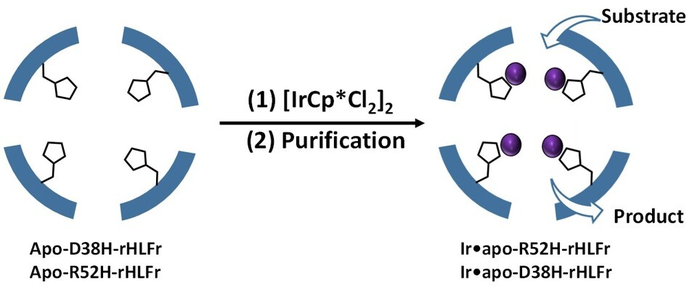 A schematic representation of enhanced iridium complex (IrCp*) uptake by the ferritin bio-nanocage. The cage was engineered with amino acid replacements by introducing site-specific mutations that allowed more IrCp* uptake. (Image Credit: Takafumi Ueno from Tokyo Institute of Technology).
A schematic representation of enhanced iridium complex (IrCp*) uptake by the ferritin bio-nanocage. The cage was engineered with amino acid replacements by introducing site-specific mutations that allowed more IrCp* uptake. (Image Credit: Takafumi Ueno from Tokyo Institute of Technology).
Their findings indicate that hybrid bio-nanocages could efficiently catalyze reactions to produce industrially essential products.
Biological polymers can suddenly self-assemble into multifaceted structures that look like cages or vessels, but are much smaller, and are known as "nano-cages." These structures can house a wide variety of molecules inside them as “guests.”
One common example is the “ferritin nanocage,” which is created by the self-assembly of 24 subunits into the protein ferritin and can enclose metal ions that are essential catalysts. Using these metal ions, a catalytic reaction can turn any substrate into a product. Although extensively known, the probable applications of the ferritin cage in industry are still to be completely explored.
So far, the majority of efforts to boost metal ion uptake in ferritin have led to cages with low stability. To ensure that the “guest” sits well inside the cage, an effective design is the answer. Considering that, a team of researchers guided by Prof. Takafumi Ueno, from the Tokyo Institute of Technology, Japan (Tokyo Tech), added site-specific mutations at the core of the ferritin nanocage and boosted its uptake of iridium complex (IrCp*).
Their findings have been published in Angewandte Chemie. Iridium is an essential catalyst in the alcohol production pathway and is used commercially in the food, pharmaceutical and chemical sectors.
Based on previous literature, we knew that the presence of coordination amino acids in the cage improve iridium activity, and that substituting these amino acids with appropriate residues could alleviate the problem. Since iridium complex behaves as a catalyst, coordination residues would do the job.
Takafumi Ueno, Professor, Tokyo Institute of Technology
The researchers used the amino acid histidine to substitute two residues, arginine and aspartic acid of the regular (wild type) ferritin cages and form the mutants R52H and D38H. Extraordinarily, the assembly structure or cage size was not influenced by these alterations.
Subsequently, they incorporated IrCp* into the mutants and discovered that R52H could embed 1.5 times more iridium atoms than the wild-type cage. However, what caught their attention was the D38H mutant, which acted precisely like the wild type. But, both the mutations did not have the same effect.
This implies that it is not only the presence of the histidine residue but also its position that is crucial to determine uptake efficiency in the cage.
Takafumi Ueno, Professor, Tokyo Institute of Technology
With the help of the new catalytic cages, the team was able to realize alcohol production rates as high as 88%. Obviously, the mutations preferred a structural re-arrangement of the reaction elements, which improved the conversion rate.
To comprehend how the substrate acted within the cage, the scientists employed simulations where the substrate molecules could travel freely inside the nanocage. They noticed some interactions between the substrate and histidine in the R52H mutant, which were not present in the wild-type cage, i.e., the substrate displayed preferential binding within the nanocage.
These hybrid bio-nanocages were also found to be highly stable, suggesting that they could be used as viable catalysts in industrial applications.
Takafumi Ueno, Professor, Tokyo Institute of Technology
The present structure-based strategy of the metal ion binding site research could be advanced to develop unique ferritin mutants with selective uptake of particular guest molecules, for diverse catalytic applications in the pharmaceutical and chemical sectors.
Journal Reference:
Taher, M., et al. (2022) Controlled Uptake of an Iridium Complex inside Engineered apo-Ferritin Nanocages: Study of Structure and Catalysis. Angewandte Chemie. doi.org/10.1002/anie.202116623.