A nano-scale structure that integrates gold, copper and silver to act as a better catalyst in a chemical reaction has recently been developed. The improved performance of the catalyst will be critical if carbon capture and utilization efforts are to be effective in assisting global warming mitigation.
 Proposed possible mechanism for the electrochemical CO2RR on (a) Au@Ag NRs and (b, c) asymmetric AuAgCu NSs. Image Credit: Nano Research.
Proposed possible mechanism for the electrochemical CO2RR on (a) Au@Ag NRs and (b, c) asymmetric AuAgCu NSs. Image Credit: Nano Research.
The research was published on March 15th, 2022, in the journal Nano Research.
In response to the threat of climate change, policymakers have increasingly focused on carbon capture and utilization (CCU), which involves drawing CO2 from the atmosphere and using it as a feedstock for industrial chemicals (like formic acid, carbon monoxide, ethylene and ethanol) or the production of carbon-neutral synthetic fuels (particularly beneficial for hard-to-electrify transport sectors like long-haul aviation and shipping).
As long as the latter process is driven by clean electricity, it also provides a long-term storage option for renewable energy — the holy grail of overcoming the intermittent nature of renewable energy sources like wind and solar power.
A chemical reaction known as the electrochemical CO2 reduction reaction (eCO2RR, or simply ECR) is one way to accomplish all of this. By separating CO2’s carbon atoms from its oxygen atoms, electricity is used to energize the conversion of the gas into other useful substances. In some types of ECR, where carbon atoms are combined with hydrogen to form various species of hydrocarbons or alcohols, water can also provide hydrogen “donors.”
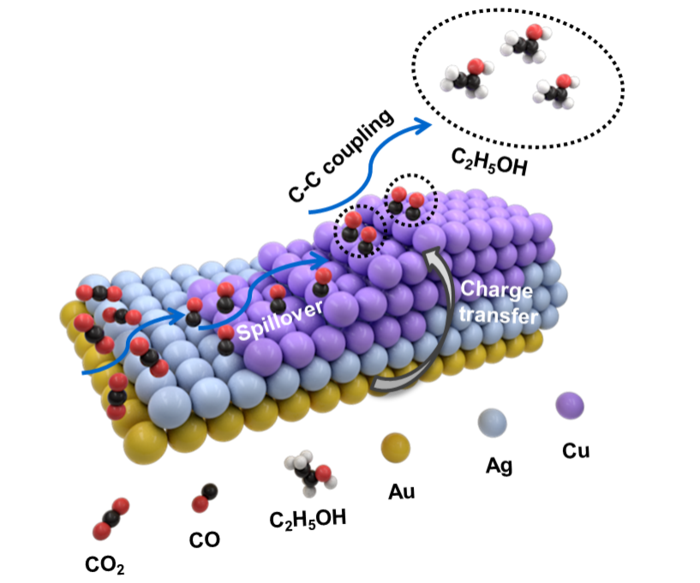
The use of the right catalyst, or chemical substance with the structure and charge to start or speed up a chemical reaction, is crucial to ECR. Various metals have been used as catalysts, depending on the desired end product. Catalysts using a single type of metal include silver to produce carbon monoxide (CO), tin for formic acid, and copper to produce methane, ethylene or ethanol.
When ECR competes with the tendency of hydrogen atoms in the electrochemical dissociation of water to pair up with themselves rather than joining up with the carbon atoms, the performance of the process can be limited. This competition may result in the production (or “selection”) of a chemical end product that is different from the one preferred. As a result, chemists have been looking for catalysts with high “selectivity” for a long time.
Rather than using a single metal as a catalyst, scientists have recently turned to heterostructures, which combine the properties of two different materials to produce different or superior results than either of the individual materials on their own.
The combination of silver and palladium in a branchlike formation (AgPd “nanodentrites”), as well as various other combinations of two metals in tube-like, sandwich-like, pyramidal and other shapes, have all been tested for ECR.
Researchers have had a lot of success with bimetallic heterostructures that include copper, which is particularly good at converting CO2 into two-carbon products. Zinc-copper (ZnCu), Silver-copper (AgCu), and gold-copper (AuCu) are examples of bimetallic heterostructures with high selectivity for carbon dioxide, methane and carbon monoxide.
We thought if two metals were producing good results, then perhaps three metals would be even better.
Zhicheng Zhang, Study Co-Author and Nanochemist, Tianjin University
As a result, the researchers created an asymmetric trimetallic nanostructure that combined silver, gold and copper. The shape and exact ratio of the three metals can be changed using a multi-step growth method. Gold “nanopyramids” are synthesized first and used as “seeds” for the subsequent growth of different trimetallic structures involving various metal ratios.
The researchers discovered that by adjusting the ratios of these three metals and the unique form of their heterostructure design, they could fine-tune the selectivity toward various C2-based products. A heterostructure with a feeding ratio of one atom each of silver and gold combined with five copper atoms was used to maximize ethanol (C2H6O) production in particular.
The study outlines a promising approach for developing other trimetallic nanomaterials in the context of ECR research.
Journal Reference:
Zhu, Y., et al. (2022) Selectivity regulation of CO2 electroreduction on asymmetric AuAgCu tandem heterostructures. Nano Research. doi.org/10.1007/s12274-022-4234-5.