Enzyme-based electrochemical biosensors combine the potentials of catalytic proteins, potentiostats, and high-tech sensor designs. In an article recently published in the journal ACS Omega, researchers constructed polyacrylic acid-deposited glucose oxidase-loaded carbon nanotube-modified platinum (PAA/GOx-CNT-modified Pt) disk electrodes as novel glucose biosensors.

Study: Cheap and Sustainable Biosensor Fabrication by Enzyme Immobilization in Commercial Polyacrylic Acid/Carbon Nanotube Films. Image Credit: Marcin Janiec/Shutterstock.com
Enzyme-Based Biosensors
Protein biocatalysts of high specificity and efficiency transform substrates into desired products. Similarly, progress in software development and electronic hardware has led to the availability of hand-held potentiostats. Device portability, sub-nanoampere current acquisition, and wireless connection to electrode array multiplexing and electrochemical sensors are now practically conceivable.
In addition, nanomaterial science and electrochemical developmental methodologies have led to reliable procedures and advanced electrodes to apply in electroanalysis.
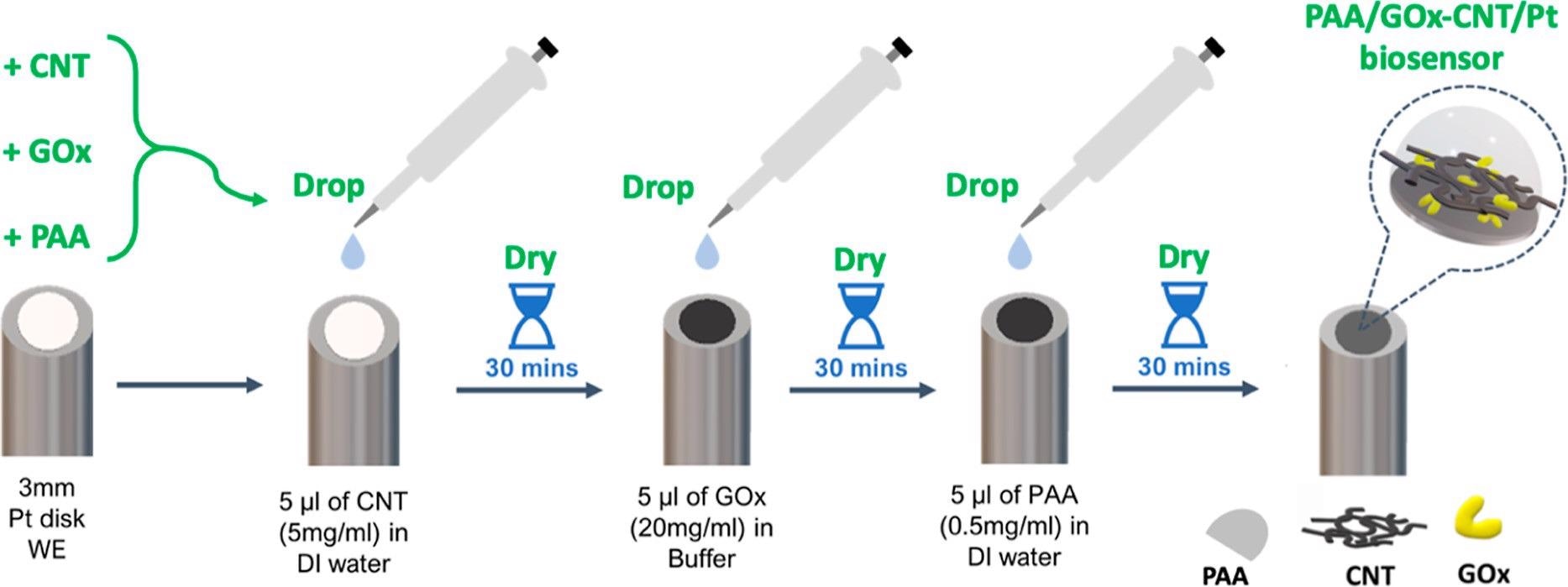
PAA/GOx-CNT/Pt glucose biosensor fabrication procedure as a simple sequence of three drop-and-dry steps with solutions or suspensions of commercial stock materials. Image Credit: Sakdaphetsiri, K et al., ACS Omega
Enzymes are composed of folded amino acid chains that are dynamic in nature. Under extreme environmental conditions, the enzymes lose their biocatalytic activities due to their denaturation or structural distortion. Thus, gentle immobilization of the analyte-specific protein onto the amperometric detector’s surface is critical to manufacture an enzyme-based biosensor. The detectors may be based on carbon disk electrodes or noble metals and are recently integrated to be a part of screen-printed electrode platforms that are mass fabricated.
The strategies involved in the fixation of functional enzymes include covalent bonding, adsorption, and entrapment behind or in semipermeable polymeric electrode coatings. So far, embedding proteins in natural or synthetic macromolecule-based thin films is the frequently used immobilization technique. PAA is used extensively in industries, and its application in the fabrication of sensors exploits the carboxylic groups on the radio frequency films.
In previous reports, PAA was used as a biocomponent with carbodiimide-assisted covalent bonding of GOx to PAA membranes, which used amperometric hydrogen peroxide (H2O2) for glucose measurements. PAA was also used in formulating an anodic electrodeposition paint (EDP) with anticorrosive properties.
Novel PAA/GOx-CNT/ Pt Biosensors
In the present study, the researchers fabricated novel glucose biosensors by loading GOx into nanospheres of CNT films on Pt disk electrodes. Further, PAA deposition helped trap enzymes on the sensor's surface. They observed an instant step in the H2O2 oxidation current in an amperometric biosensor integrated with anodic H2O2 detection at a potential of +600 millivolts.
The fabricated biosensors could reliably determine the glucose concentrations in beverage and human serum. These Pt electrode disks used in the present novel biosensor offer a unique combination of two cheaply available matrix constituents, and the sensor layer is formed through drop and dry steps. PAA/GOx-CNT/ Pt biosensors fabricated are user-friendly and green bioanalytical tools that are cost-effective, and their production does not need special skills or laboratory amenities.
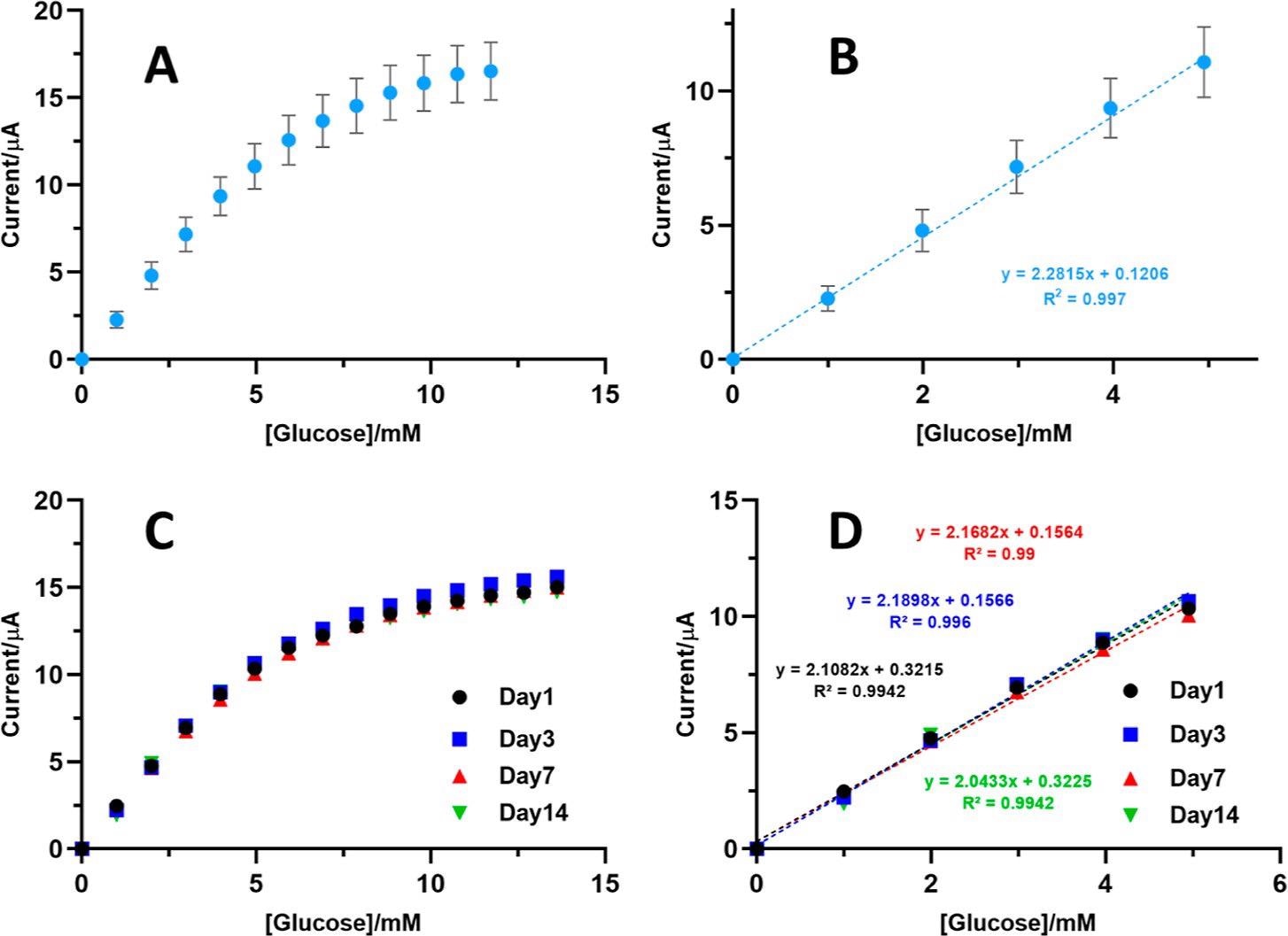
PAA/GOx-CNT/Pt glucose biosensors: fabrication reproducibility and response stability. (A) Averaged amperometric calibration plot constructed with data from day 1 glucose response testing of eight biosensor prototypes, prepared identically on different days. (B) Zoomed view of the linear range of the calibration plot in (A) with results from linear regression analysis. (C) Amperometric calibration plots of a single biosensor prototype, on day 1 (black dots), day 3 (blue squares), day 7 (red triangles), and day 14 (green triangles) after preparation, with storage in refrigerated 0.1 M Na-PB between individual assessments. (D) Linear region for day 1, 3, 7, and 14 calibration plots in (C). Experimental parameters for the amperometry trials were as in Figure 2. Image Credit: Sakdaphetsiri, K et al., ACS Omega
Research Findings
The researchers carried out a control experiment with GOx-CNT/Pt biosensors that lacked PAA protective layer by storing them in sodium phosphate buffer (Na-PB). The results from the amperometric sensor revealed that within 30 minutes, the biosensors lost about 75% of glucose response, while longer storage of biosensors in Na-PB did not affect the current response. They interpreted that about three-quarters of totally entrapped GOx in the CNT matrix was loosely trapped and could diffuse into the bulk solution.
Before analyzing real blood samples and beverages, the researchers used PAA/GOx-CNT/Pt biosensors to determine glucose in Na-PB of known content. The first analytical task was performed with three biosensors that were similarly prepared, and the results showed that biosensor copies reached sample content recoveries of 99.3 ± 0.8, 101.3 ±1.4, and 104.1± 0.6%, with the average recovery performance of 101.6 ± 2.2%. This consistency in measuring the glucose content of the three tested biosensors suggested the usefulness of PAA/GOx-CNT/Pt biosensors and confirmed the reliability in biocatalytic response and reproducibility of fabrication.
The team also confirmed the analytical performance with Na-PB model samples by operating the PAA/GOx-CNT/Pt biosensors to evaluate the glucose content in blood serum and carbonated beverage Pepsi.
Parallelly, the glucose content in serum and Pepsi was analyzed with a commercial blood glucose meter and refractive index detector integrated with high-pressure liquid chromatography. The results revealed that the glucose concentrations and recovery rate percentage for serum and Pepsi glucose were 5.8 ± 0.1 and 240.3 ± 16.0 millimoles and 97.5 ± 2.2 and 98.7 ± 6.6%, respectively.
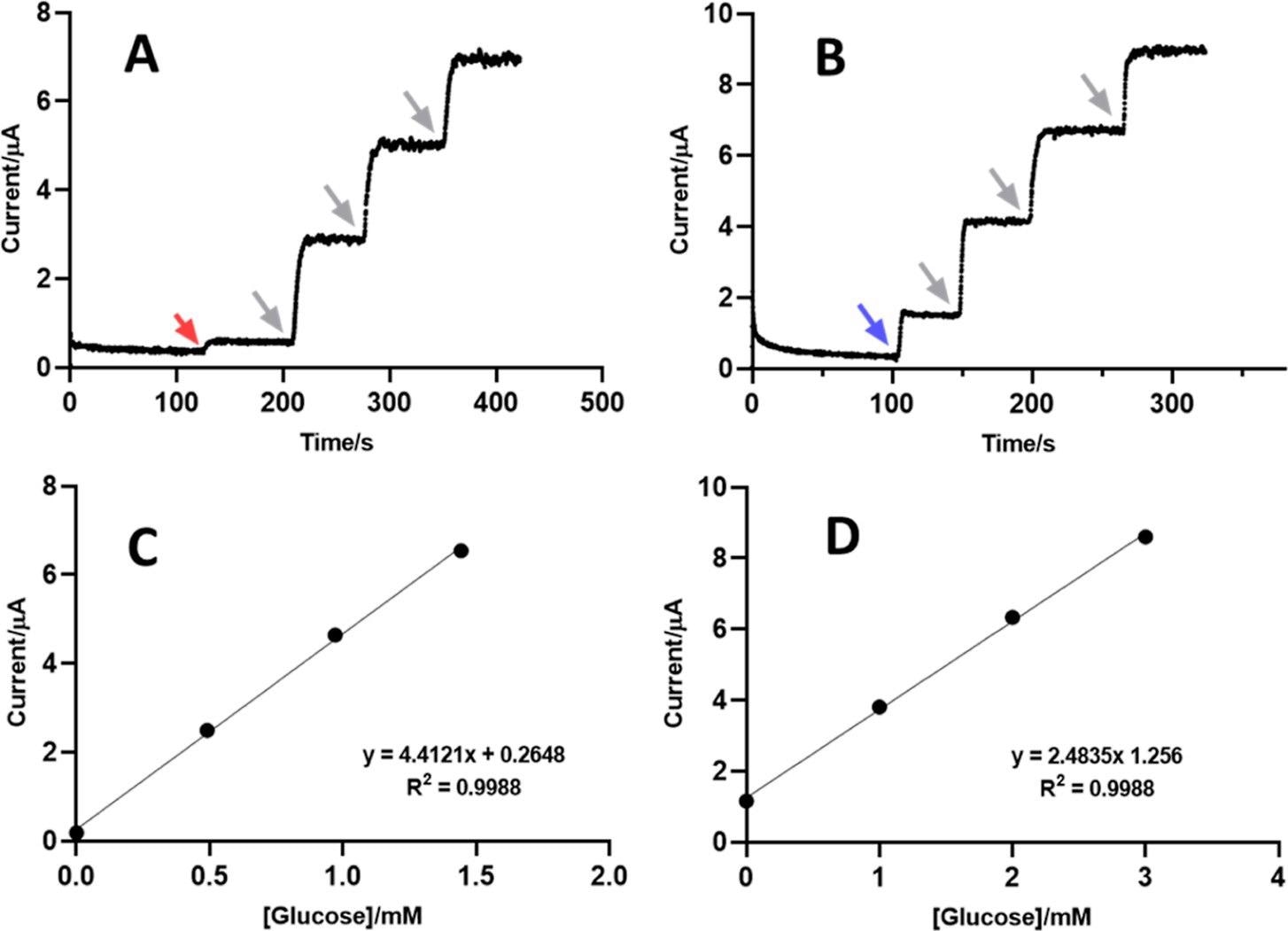
Application of PAA/GOx-CNT/Pt for real sample glucose testing. (A,B) Amperograms and (C,D) standard addition plots as obtained for the analysis of glucose in blood serum (A,C) and Pepsi (B,D) in standard addition mode. Addition of samples to trial buffer (red arrow, serum; blue arrow, Pepsi) was followed by three additions of small aliquots of glucose stock solution (gray arrows). Electrolyte for the measurements was 0.1 M Na-PB, and amperometry was at 25 °C, with an anodic H2O2 detection potential of + 0.6 V vs RE. Experimental parameters for the amperometry trials were as in Figure 2. Image Credit: Sakdaphetsiri, K et al., ACS Omega
Conclusion
In conclusion, the authors reported the construction of a robust amperometric glucose biosensor, where GOx was the target-specific biocatalyst that was immobilized onto Pt disk working electrode by entrapping GOx in CNT-based nanopores.
Once loaded, the polymeric PAA layer superimposition prevented the escape of GOx, thus sealing the pore exits. The team observed a fast current response of PAA/GOx-CNT/Pt biosensors, which was proportional to glucose concentration. The proposed PAA/CNT-based biosensors required cost-efficient and commercially available materials, suggesting that the current strategy abides by the principles of green chemistry. Moreover, the simple preparation procedure of the PAA/GOx-CNT/Pt biosensor confirms its potential application across the disciplines of health and science.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Sakdaphetsiri, K., Teanphonkrang, S., and Schulte, A. (2022). Cheap and Sustainable Biosensor Fabrication by Enzyme Immobilization in Commercial Polyacrylic Acid/Carbon Nanotube Films. ACS Omega. https://pubs.acs.org/doi/full/10.1021/acsomega.2c00925