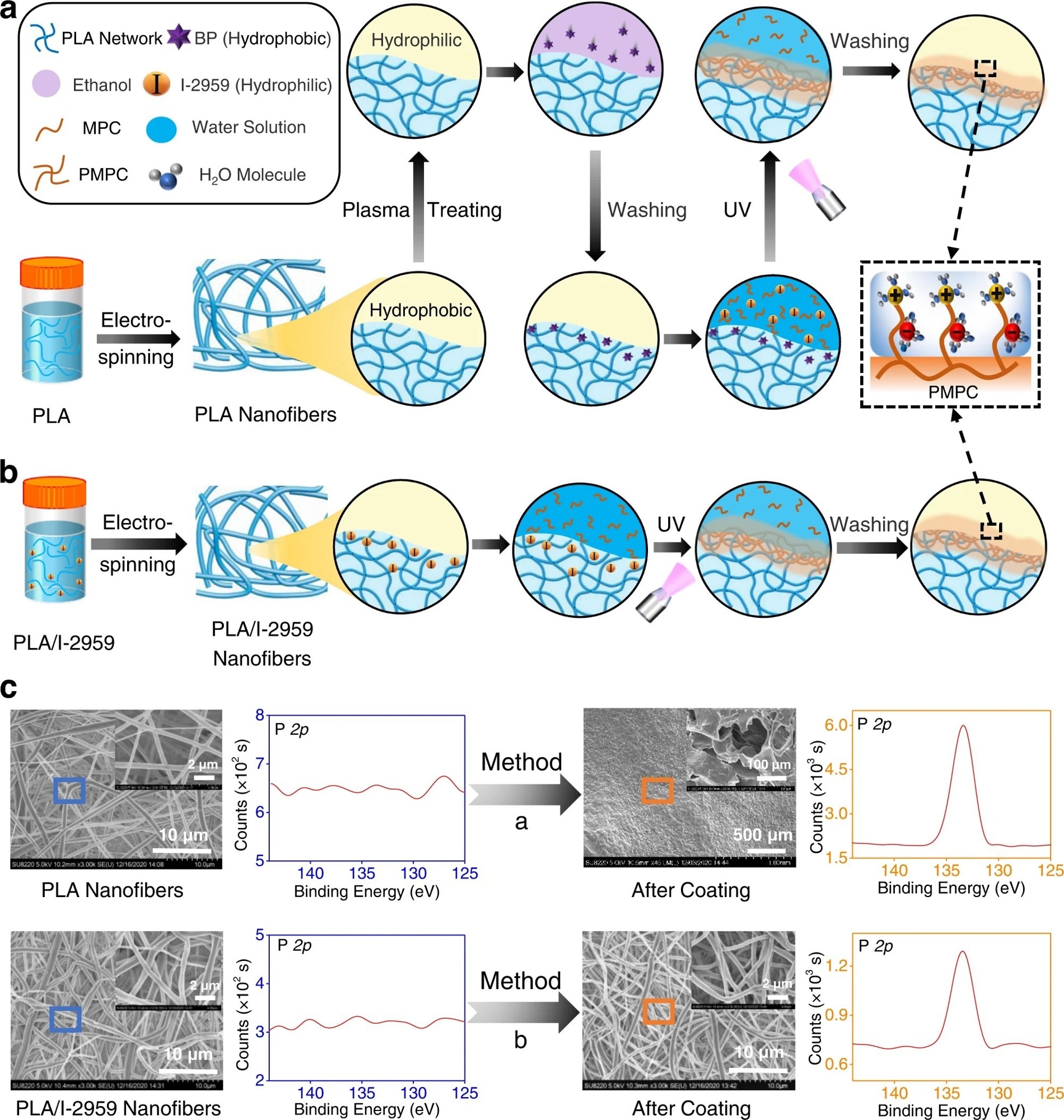
Figure 1. The comparison of our developed surface coating strategy to construct superlubricated nano-skin on electrospun nanofibers with previously reported method. a Schematic diagram of a slightly changed procedure from previous method17,18. PLA polylactic acid, BP benzophenone, MPC 2-methacryloyloxyethyl phosphorylcholine, PMPC poly MPC, UV ultraviolet. b Schematic diagram of our optimized method of subsurface-initiated polymerization. c Representative SEM and XPS results of electrospun nanofibers for the comparison of surface morphology and elemental composition between the method in a and in b. The experiments are replicated three times independently with similar results. © Wang, Y., Xu, Y., Zhai, W. et al. (2022)
These nanofibrous membranes are also used for in vivo treatments that require direct human tissue contact. The effectiveness of the application of electrospun nanofibers depends on their surface performance. The electrospun nanofiber's surface properties, such as patterned structure and fiber orientation, are effectively adjusted via various methods.
The incorporation of these properties improves the cell growth capacity on the fiber surface along with cellular adhesion, which plays an important role in the acceleration of the tissue regeneration process. Nevertheless, uncontrolled cell growth and adhesion to adjacent tissues lead to irretrievable consequences.
Non-Specific Cell Adhesion Property of Electrospun Membranes
Electrospun polylactic acid (PLA)-based membranes (e.g., DK-film) are clinically used for anti-adhesion purposes. This membrane forms a barrier between the injured tissues. However, one of the disadvantages of this membrane is that it adheres to the tissue surface. Therefore, it is extremely crucial to create an electrospun membrane with superior non-adhesive surface properties to prevent post-operative adhesion.
Hydration lubrication could be effectively used to develop nanofibrous membranes with non-adhesive surfaces. Mechanistically, polyelectrolyte polymers (e.g., poly (2-methacryloyloxyethyl phosphorylcholine) (PMPC)) exhibit strong adsorption to the hydration layer due to the zwitterionic charges and, subsequently, promote an extremely low coefficient of friction (COF) between the sliding surfaces. This condition prevents non-specific cell adhesion.
Optimization of the interfacial bonding between the substrate polymer chains of nanofibers and the zwitterionic polymer chains in the coating is a challenging task. Although surface modification methods (e.g., grafting polymer chains) are used for this purpose, they cannot develop strong zwitterionic coatings. This is because PLA is sensitive to organic solvents and gets dissolved during surface modification.
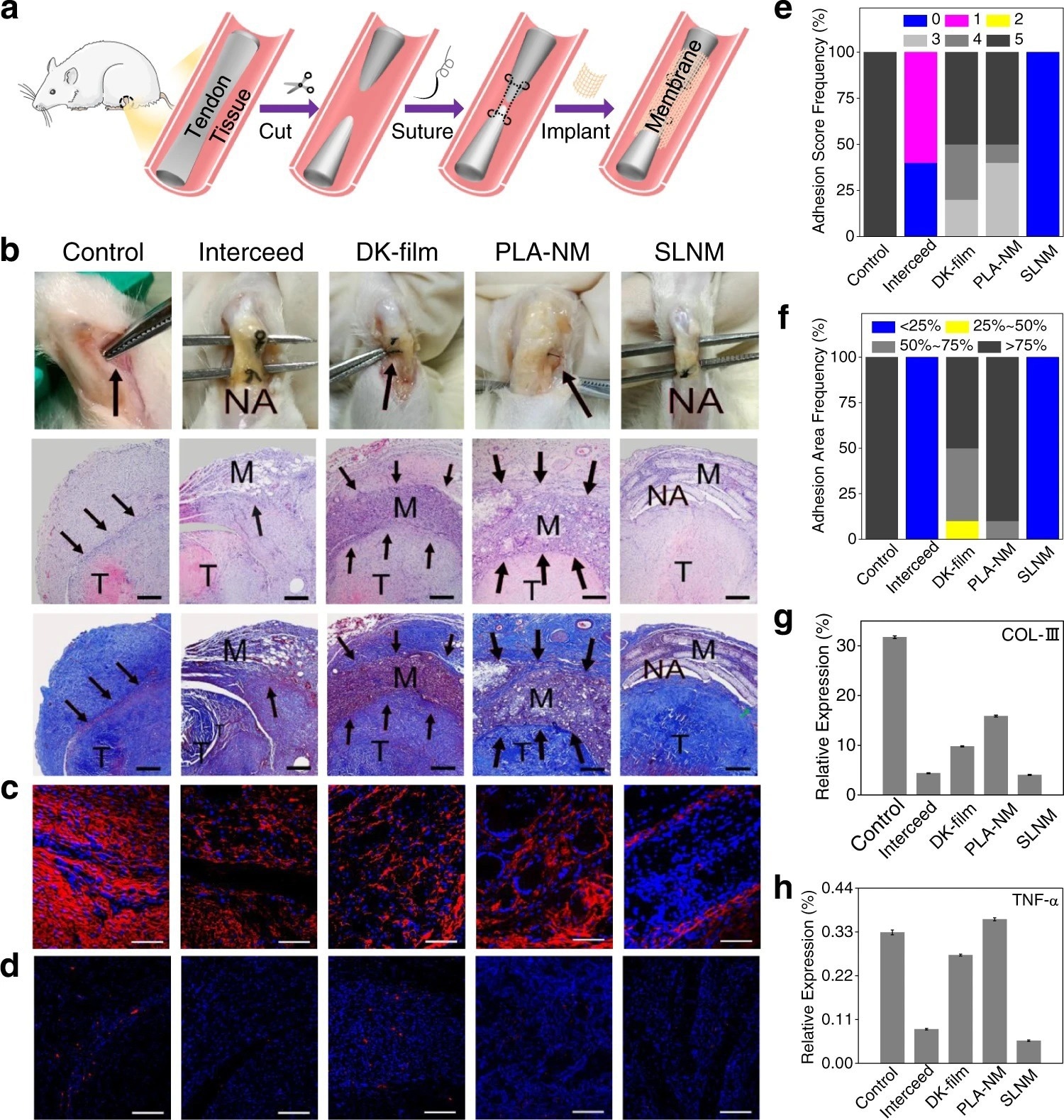
Figure 2. In vivo antitissue adhesion properties of the superlubricated electrospun nanofibrous membranes based on rat tendon adhesion model. a Schematic diagram showing the overall animal test process. b Photos of the harvested tendon on 14 d following implantation and H&E staining as well as Masson staining images. Scale bar: 500 µm. The black arrows point to adhesion site. M membrane. T tendon. NA no adhesion. Representative confocal laser scanning microscopic images for the immunofluorescent staining of c COL-III (scale bar: 100 µm) and d TNF-α (scale bar: 200 µm). Red color represents targeted protein and blue color represents cell nucleus. Comparison of e Adhesion score, f Adhesion area, and relative expression levels of g COL-III as well as h TNF-α for the control, Interceed, DK-film, PLA-NM, and SLNM groups, respectively. The experiments in b–d are replicated three times independently with similar results. © Wang, Y., Xu, Y., Zhai, W. et al. (2022)
Development of Superlubricated Nano-skin on Electrospun Nanofibers
Hydrogel skin that cross-links with the substrate and promotes a tough interfacial bonding was recently developed. A similar method was implemented to develop superlubricated nano-skin on electrospun nanofibers. In this study, benzophenone (a hydrophobic initiator) was used to soak the subsurface of electrospun PLA nanofibers. Before this treatment, the electrospun PLA nanofibers were treated with plasma.
In a previous method, after soaking in benzophenone, the electrospun nanofibrous membranes were immersed in an aqueous solution of methacryloyloxyethyl phosphorylcholine (MPC) monomer, which is a hydrophilic initiator 2-hydroxy-1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-1-propanone (I-2959). The next step was to expose to ultraviolet light, for 30 minutes, on each side. The membrane was rinsed with enough deionized water to remove the weakly linked PMPC molecules. The newly synthesized surface-functionalized electrospun PLA nanofibers containing PMPC on the surface exhibited hydration lubrication performance.
Recently, in situ superlubricated nano-skin (SLNS) was grown (inside-out) on an electrospun nanofiber surface. During the electrospinning process, I-2959 (hydrophilic small molecules) self-arranged in the subsurface of the PLA nanofibers (hydrophobic polymer). The main advantage of this technique is that the second initiator outside the nanofibers is not required. As stated above, photopolymerization was performed by subjecting both sides of the electrospun PLA/I-2959 nanofibrous membranes to ultraviolet rays for 30 minutes, which was then immersed in an aqueous MPC monomer solution. The electrospun nanofiber membrane was rinsed with deionized water to remove unbound MPC and, thereby, superlubricated membranes were synthesized.
Both the techniques described were compared using various analytical tools, such as X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy (SEM). The surface elemental compositions of the newly synthesized superlubricated membranes were analyzed using XPS.
The electrospun nanofiber synthesized using the first method showed the presence of a phosphorous element, which originates from PMPC. Hence, XPS data indicated that PMPC coating was successfully prepared in the first method. However, a SEM analysis showed prominent damage to nanofibers, which strongly suggests that hydrogel-skin coating cannot be applied to nanofibers. Nevertheless, SEM and XPS data of the newly developed superlubricated nano-skin (SLNS) showed successful coating without destroying nanofiber structures.
Importantly, the in situ grown superlubricated coating formed on the electrospun nanofiber surface with a thickness of around 1 to 10 nm. Additionally, the COF was lower than 0.025. The newly developed nanofibrous membranes exhibited ideal tensile property and biocompatibility.
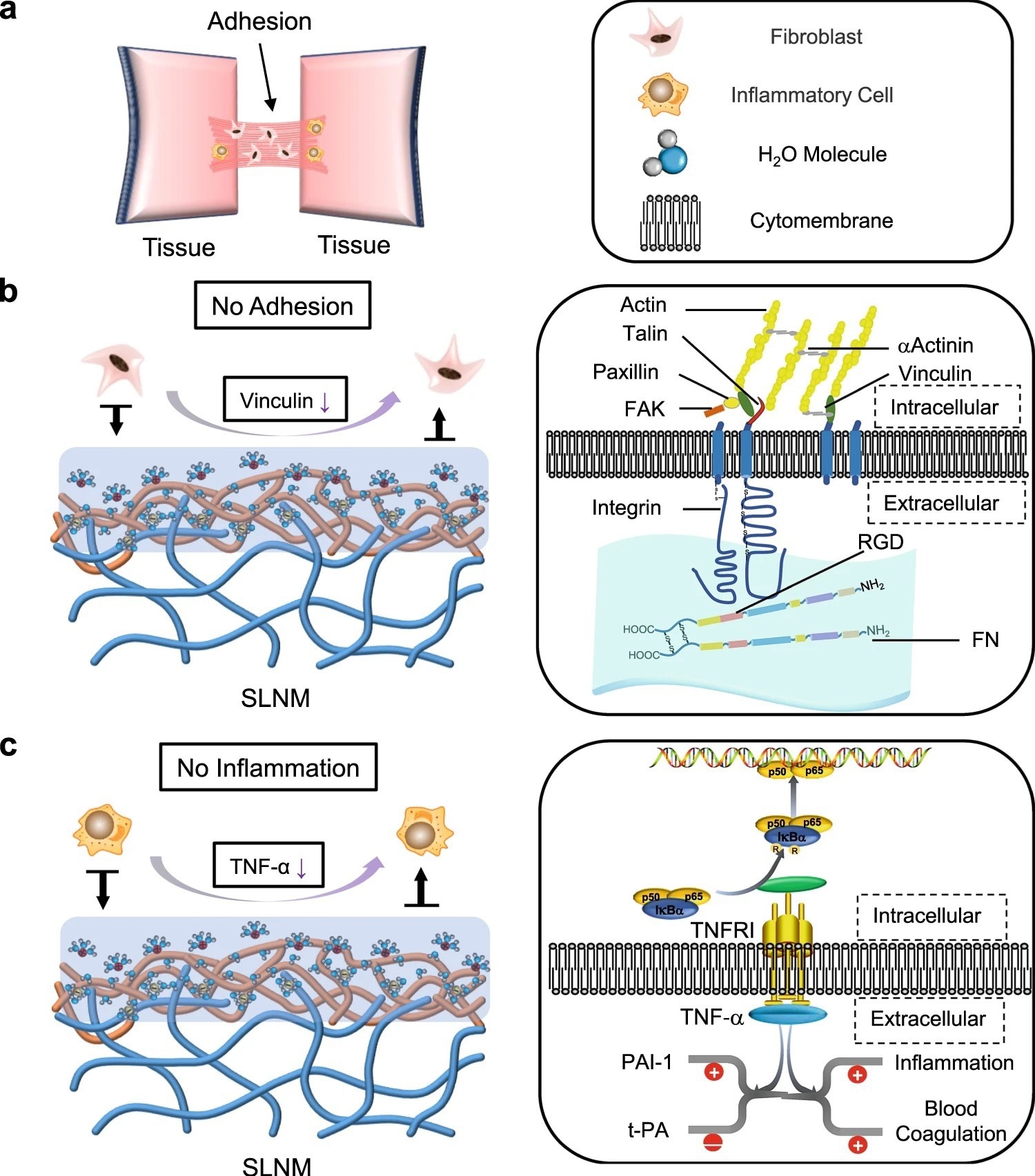
Figure 3. Potential antiadhesion mechanism of the superlubricated electrospun nanofibrous membranes. a Schematic diagram showing the occurrence of postoperative tissue adhesion. Interstitial fibrosis and inflammation are involved in this process. Schematic diagrams showing the mechanisms of b inhibiting fibrosis and c reducing inflammation based on the tenacious hydration layer formed surrounding the zwitterionic phosphorylcholine groups on the SLNM surface. © Wang, Y., Xu, Y., Zhai, W. et al. (2022)
Anti-adhesion Performance of Superlubricated Nano-Skin on Electrospun Nanofibers
The newly developed SLNM with the superlubricated nano-skin exhibited significant anti-adhesion performance. Importantly, compared to two commercially used anti-adhesion products, namely, DK-film and intercede, the newly synthesized material showed greater anti-adhesion performance with a lower production cost. This finding was validated using an in vitro anti-cell adhesion test and an in vivo study (anti-tissue adhesion test) was also performed using rat abdominal adhesion and tendon adhesion models. In the future, the application of the newly developed superlubricated biomaterial could prevent post-operative adhesion.
Reference
Wang, Y., Xu, Y., Zhai, W. et al. (2022) In-situ growth of robust superlubricated nano-skin on electrospun nanofibers for post-operative adhesion prevention. Nature Communications, 13, 5056. https://www.nature.com/articles/s41467-022-32804-0
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.