Reviewed by Lexie CornerApr 22 2024
In a recent paper published in Chem, researchers from Pohang University of Science and Technology have developed conducting two-dimensional polymers with electron mobility comparable to graphene. These polymers offer promising potential for applications requiring high electrical conductivity.
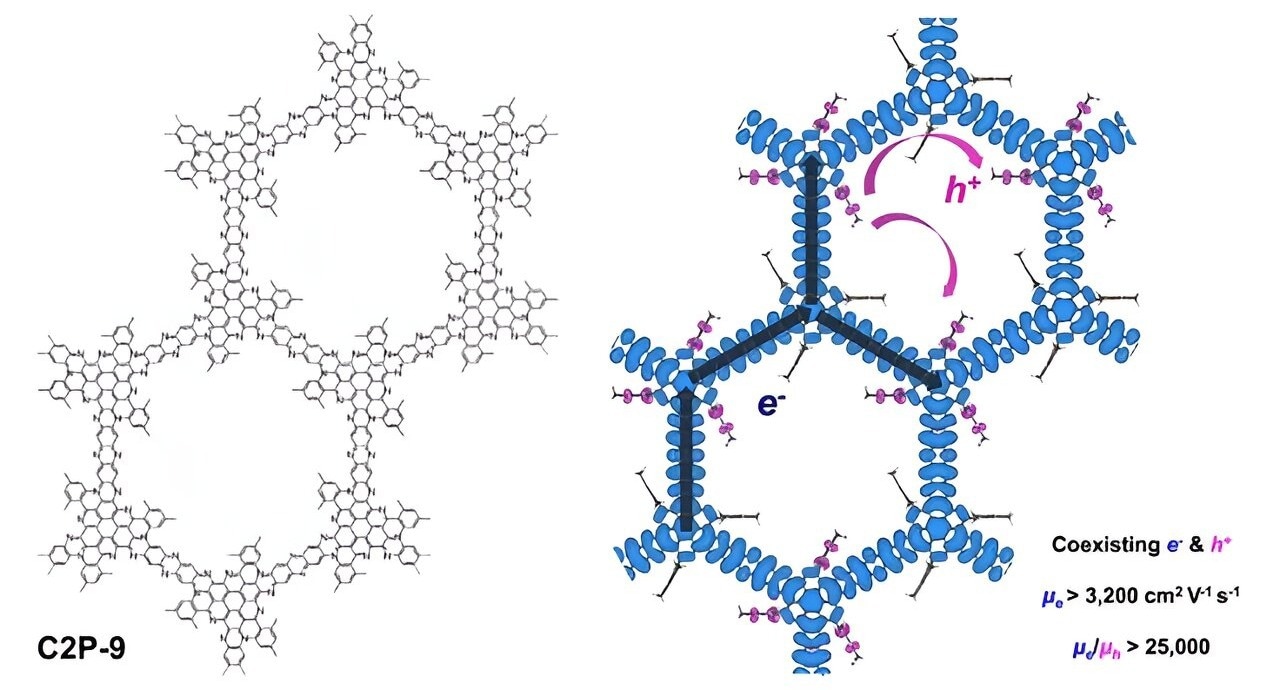 Diagram depicting the chemical structure of a conducting two-dimensional polymer (C2P-9) with pendant groups and the coexistence of ultrafast electrons after p-type doping. Image Credit: Pohang University of Science and Technology
Diagram depicting the chemical structure of a conducting two-dimensional polymer (C2P-9) with pendant groups and the coexistence of ultrafast electrons after p-type doping. Image Credit: Pohang University of Science and Technology
Graphene, known as a “dream material,” has a strength 200 times greater than steel and an electron mobility 140 times faster than silicon. However, its inability to have a band gap, which is necessary to control electrical current, precludes its application as a semiconductor. Researchers have been diligently investigating diverse methodologies to fabricate a semiconductor that exhibits graphene's remarkable characteristics.
Developing conducting polymers is one promising strategy. To achieve remarkable qualities, scientists are investigating conducting polymers with a fused aromatic backbone that resembles the chemical structure of graphene.
However, interlayer stacking between growth intermediates causes problems during synthesis, impeding appropriate polymer growth.
The team, led by Professors Kimoon Kim, Ji Hoon Shim, and Dr. Yeonsang Lee from the Department of Chemistry at Pohang University of Science and Technology (POSTECH), used triazacoronene, which has a chemical structure similar to graphene, and added bulky pendant functional groups to its periphery.
This study was conducted by Professor Jun Sung Kim from the Department of Physics at POSTECH and the Center for Artificial Low-Dimensional Electronic Systems at the Institute for Basic Science.
The group was able to suppress the stacking of two-dimensional polymer intermediates during the polymerization of triazacoronene monomers by introducing steric hindrance from these pendant groups.
P-type doping enhanced intermediate solubility and made it easier to synthesize two-dimensional polymers with a higher degree of polymerization and fewer defects, producing exceptional electrical conductivity.
According to magnetotransport measurements, in contrast to hole-carrier transport, which has 25,000 times lower mobility at low temperatures, coherent multi-carrier transport with finite n-type carriers exhibits remarkably high mobility over 3,200 cm2 V−1 s−1 and long phase coherence length exceeding 100 nm.
Dispersive and flat bands make up the spatially separated electronic states close to the Fermi level and are responsible for this sharp difference in electron and hole-carrier transport.
We have achieved a breakthrough in addressing low electron mobility, a major challenge in organic semiconductors, and in controlling the conduction pathways for electrons and holes at the molecular level, this research shed light on enhancing material performance across various industrial applications including batteries and catalysts.
Kimoon Kim, Professor, Department of Chemistry, Pohang University of Science and Technology
Journal Reference:
Lee, Y., et al. (2024) Observation of ultrafast electrons in pendant-embedded conducting two-dimensional polymers. Chem. doi.org/10.1016/j.chempr.2023.12.007