Reviewed by Lexie CornerJun 5 2025
Researchers at Southern Medical University developed a self-propelled ferroptosis nanoinducer that can reach deeper into tumor tissues. This led to better anti-cancer effects while maintaining good biocompatibility.
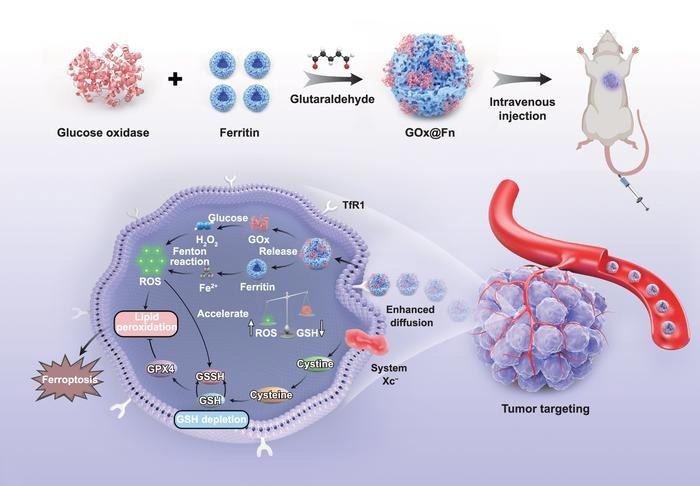 Fabrication and in vivo mechanism of self-propelled ferroptosis nanoinducer for tumor targeting. Image Credit: By Wenxin Xu, Hao Tian, Yanzhen Song, Hanfeng Qin, Junbin Gao, Yichi Chen, Weichang Huang, Lin Lin, Haixin Tan, Yicheng Ye, Xiaoting Zhang, Daniela A Wilson, Guang Yang, Fei Peng and Yingfeng Tu
Fabrication and in vivo mechanism of self-propelled ferroptosis nanoinducer for tumor targeting. Image Credit: By Wenxin Xu, Hao Tian, Yanzhen Song, Hanfeng Qin, Junbin Gao, Yichi Chen, Weichang Huang, Lin Lin, Haixin Tan, Yicheng Ye, Xiaoting Zhang, Daniela A Wilson, Guang Yang, Fei Peng and Yingfeng Tu
The study supports further development of biocompatible nanotherapeutics with multiple functions for cancer treatment.
Limited penetration into tumor tissue remains a challenge in advancing nanotherapeutic approaches for cancer.
Conventional nanoplatforms cannot achieve active penetration, leading to poor penetration depth and efficiency into tumor tissues. It might weaken the tumor inhibitory effect of the nanoplatform. Here we are saying, why not design a nanotherapeutic that can actively penetrate deeper into tumor tissues via enhanced diffusion?
Yingfeng Tu, Study Corresponding Author and Professor, School of Pharmaceutical Sciences, Southern Medical University
Cancer remains a major health concern, with increasing mortality rates and rising treatment costs. Standard treatments like surgery, radiotherapy, and chemotherapy are often associated with significant side effects.
Ferroptosis is a recently identified form of programmed cell death and plays a role in tumor development. In response, researchers have begun designing ferroptosis-based nanoplatforms for cancer therapy. However, these approaches face challenges such as poor biocompatibility, limited tumor penetration, and low loading of active pharmaceutical ingredients (APIs).
To address these issues, Tu and colleagues used glutaraldehyde as a crosslinking agent to develop active nanoparticles composed of two natural proteins: glucose oxidase and ferritin. The resulting self-propelled nanotherapeutics showed improved diffusion and deeper tumor penetration.
The combination of the two proteins triggered ferroptosis inside cells, leading to membrane breakdown and damage to multiple tumor cell organelles.
The research team studied the nanoinducer over a two-year period. They analyzed its structure, movement, and chemotactic behavior. They also assessed the tumor-inhibitory effectiveness of the produced nanotherapeutic in vitro and in vivo.
Tu added, “Biocompatibility is an issue that deserves greater attention. With the pure-protein framework, potential systemic toxicity can be minimized. The self-propelled nanotherapeutic we developed is capable of deeper tumor penetration with negligible toxicity at the same time. We believe this platform holds strong potential for cancer treatment.”
The researchers are continuing their work to test the nanoinducer's tumor-inhibiting effects on other cancer types, including non-small cell lung cancer. They are also working to support its potential development for clinical use.
Journal Reference:
Xu, W., et al. (2025). Self-propelled Ferroptosis Nanoinducer for Enhanced Cancer Therapy. International Journal of Extreme Manufacturing. doi.org/10.1088/2631-7990/ada838.