Aug 29 2008
Changes in protein interactions at the synapse - or junction between neurons - play a key role in many important processes during brain development, such as synapse maturation, learning and memory formation. A team of scientists, led by Katsuhiko Mikoshiba at the RIKEN Brain Science Institute in Wako, has identified how synaptic activity can regulate the binding of an adaptor protein, Homer3, to one of its targets, a membrane receptor for the neurotransmitter glutamate called metabotropic glutamate receptor 1a (mGluR1a)*.
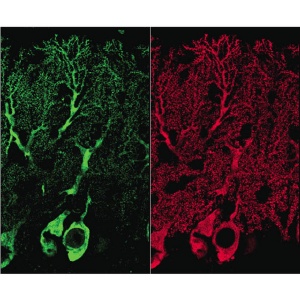 Phosphorylation alters Homer3 distribution in Purkinje cells of young mice. Homer3 protein (red) is distributed throughout Purkinje cells. By contrast, phosphorylated Homer3 (green) is preferentially distributed in soma, proximal dendrites, and some parts of postsynaptic regions in Purkinje cells.
Phosphorylation alters Homer3 distribution in Purkinje cells of young mice. Homer3 protein (red) is distributed throughout Purkinje cells. By contrast, phosphorylated Homer3 (green) is preferentially distributed in soma, proximal dendrites, and some parts of postsynaptic regions in Purkinje cells.
Synaptic activity results in an influx of calcium ions into the neuron, which turns on an enzyme called calcium/calmodulin-dependent protein kinase II (CaMKII). The researchers showed that active CaMKII chemically modifies Homer3 by adding phosphate groups onto the protein in a type of neuron known as the Purkinje cell (Fig. 1). This phosphorylation reaction causes Homer3 to be expelled from the synapse and into the cytoplasm of the neuron, which may serve to communicate the activation state of that synapse to the rest of the cell.
Akihiro Mizutani, the first author of the paper, believes that Homer3 phosphorylation “triggers the reorganization of the molecular architecture at the synapse, which can be a synaptic tag for long-lasting synaptic plasticity.”
Homer3 usually binds to mGluR1a at the membrane, coupling this glutamate receptor to another protein that regulates intracellular calcium stores. Mikoshiba and his colleagues found that phosphorylation of Homer3 inhibits its binding to mGluR1a. This switch in the Homer3-mGluR1a interaction was functionally important, because cells in which this switch was activated had a different pattern of calcium release from intracellular stores in response to glutamate than cells in which the switch was turned off.
One important message from this work is that synaptic activity can very quickly induce changes in molecular interactions, which can translate to changes in calcium signaling inside the cell. An increase in intracellular calcium at the synapse is a key step in altering the strength of synaptic connections, which is thought to be necessary for the formation of new memories.
Because the work was performed in Purkinje cells from young mice within the cerebellum, the part of the brain involved in motor learning, the findings suggest that the regulation of Homer3 phosphorylation may be involved in this important process during development.
If this mechanism is also operating in other areas of the brain and in older animals as well, the findings may also be more widely relevant to other forms of learning.
* Mizutani, A., Kuroda, Y., Futatsugi, A., Furuichi, T. & Mikoshiba, K. Phosphorylation of Homer3 by calcium/calmodulin-dependent kinase II regulates a coupling state of its target molecules in Purkinje cells. The Journal of Neuroscience 28, 5369 –5382 (2008).