Jan 22 2009
Everyone prefers to avoid the frustration of failing to achieve a desired outcome. Now it seems this even applies to materials and the arrangement of their atomic magnets, referred to as spins. Researchers from RIKEN's Nishina Center for Accelerator-Based Science in Wako, in collaboration with researchers from the University of Hyogo and Kyoto University, have uncovered an intriguing interplay between the arrangement of atomic spins and atomic interactions in the metallic compound Mo3Sb7.
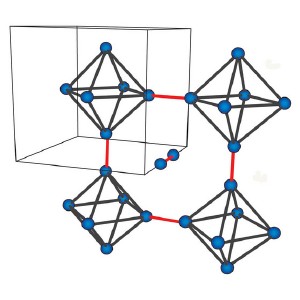 The octrahedral arrangement of Mo atoms within the crystal structure of Mo3Sb7. Strongly coupled Mo atoms at the tips of the octahedra form dumbbells (red lines) throughout the structure.
The octrahedral arrangement of Mo atoms within the crystal structure of Mo3Sb7. Strongly coupled Mo atoms at the tips of the octahedra form dumbbells (red lines) throughout the structure.
The molybdenum (Mo) atoms in Mo3Sb7 crystals are arranged in octahedra. Unusually, the atomic bonds between the Mo atoms at the tips of the octahedra are stronger than between the Mo atoms in the plane. This leads to the formation of ‘dumbbells’ of Mo pairs along the three main crystal directions. “The unusual arrangement between the dumbbells and the other Mo atoms makes this material unique and interesting to study,” comments Isao Watanabe from the research team.
Of particular interest is a sudden structural change that occurs at temperatures below 50 K (-223.15 °C). The origin of this phase transition has now been unveiled by a number of experiments that probe the magnetic and electric properties of the Mo atoms*. These measurements present clear evidence that the phase transition is accompanied by symmetry changes in the crystal.
The symmetry changes are triggered by the spins associated with Mo atoms. The researchers found that an unusual competition in interaction between the Mo atoms takes place. At the phase transition, the strength of the interaction between the Mo atoms in the octahedra becomes comparable to the bond between the Mo atoms in the dumbbells. Consequently, the Mo atomic spins then begin to arrange in an up-and-down fashion along the entire crystal rather than within the dumbbells. However, owing to the particularities of the three-dimensional crystal structure, a periodic up-and-down arrangement, which is homogeneous across the entire crystal, is impossible. Frustration is the result.
To break this frustration the Mo octahedra elongate in one direction, breaking the crystal symmetry. This in turn finally allows the Mo dumbbells to order themselves periodically throughout the crystal in an arrangement termed a ‘valence bond crystal’.
The unusual competition of the atomic bonds between the Mo atoms in Mo3Sb7 has led to dramatic consequences involving crystallographic, electronic and magnetic properties. “This is the first example of its kind and we expect that this opens a new field to study similarly complex systems,” say Watanabe and his colleagues.
- Koyama, T., Yamashita, H., Takahashi, Y, Kohara, T., Watanabe, I., Tabata, Y. & Nakamura, H. Frustration-induced valence bond crystal and its melting in Mo3Sb7. Physical Review Letters 101, 126404 (2008).