DOI :
10.2240/azojono0111
Mar 25 2006
The method by which a drug is delivered can have a significant effect on its efficacy. Some drugs have an optimum concentration range within which maximum benefit is derived, and concentrations above or below this range can be toxic or produce no therapeutic benefit at all. On the other hand, the very slow progress in the efficacy of the treatment of severe diseases, has suggested a growing need for a multidisciplinary approach to the delivery of therapeutics to targets in tissues. From this, new ideas on controlling the pharmacokinetics, pharmacodynamics, non-specific toxicity, immunogenicity, biorecognition, and efficacy of drugs were generated. These new strategies, often called drug delivery systems (DDS), are based on interdisciplinary approaches that combine polymer science, pharmaceutics, bioconjugate chemistry, and molecular biology.
To minimize drug degradation and loss, to prevent harmful side-effects and to increase drug bioavailability and the fraction of the drug accumulated in the required zone, various drug delivery and drug targeting systems are currently under development. Among drug carriers one can name soluble polymers, microparticles made of insoluble or biodegradable natural and synthetic polymers, microcapsules, cells, cell ghosts, lipoproteins, liposomes, and micelles. The carriers can be made slowly degradable, stimuli-reactive (e.g., pH- or temperature-sensitive), and even targeted (e.g., by conjugating them with specific antibodies against certain characteristic components of the area of interest). Targeting is the ability to direct the drug-loaded system to the site of interest. Two major mechanisms can be distinguished for addressing the desired sites for drug release: (i) passive and (ii) active targeting. An example of passive targeting is the preferential accumulation of chemotherapeutic agents in solid tumors as a result of the enhanced vascular permeability of tumor tissues compared with healthy tissue. A strategy that could allow active targeting involves the surface functionalization of drug carriers with ligands that are selectively recognized by receptors on the surface of the cells of interest. Since ligand–receptor interactions can be highly selective, this could allow a more precise targeting of the site of interest.
Controlled drug release and subsequent biodegradation are important for developing successful formulations. Potential release mechanisms involve: (i) desorption of surface-bound /adsorbed drugs; (ii) diffusion through the carrier matrix; (iii) diffusion (in the case of nanocapsules) through the carrier wall; (iv) carrier matrix erosion; and (v) a combined erosion /diffusion process. The mode of delivery can be the difference between a drug’s success and failure, as the choice of a drug is often influenced by the way the medicine is administered. Sustained (or continuous) release of a drug involves polymers that release the drug at a controlled rate due to diffusion out of the polymer or by degradation of the polymer over time. Pulsatile release is often the preferred method of drug delivery, as it closely mimics the way by which the body naturally produces hormones such as insulin. It is achieved by using drug-carrying polymers that respond to specific stimuli (e.g., exposure to light, changes in pH or temperature).
For over 20 years, researchers have appreciated the potential benefits of nanotechnology in providing vast improvements in drug delivery and drug targeting. Improving delivery techniques that minimize toxicity and improve efficacy offers great potential benefits to patients, and opens up new markets for pharmaceutical and drug delivery companies. Other approaches to drug delivery are focused on crossing particular physical barriers, such as the blood brain barrier, in order to better target the drug and improve its effectiveness; or on finding alternative and acceptable routes for the delivery of protein drugs other than via the gastro-intestinal tract, where degradation can occur.
Drug Delivery Systems
The global market for advanced drug delivery systems was more than €37.9 billion in 2000 and is estimated to grow and reach €75B by 2005 (i.e., controlled release €19.8B, needle-less injection €0.8B, injectable/impantable polymer systems €5.4B, transdermal €9.6B, transnasal €12.0B, pulmonary €17.0B, transmucosal €4.9B, rectal €0.9B, liposomal drug delivery €2.5B, cell/gene therapy €3.8B, miscellaneous €1.9B). Developments within this market are continuing at a rapid pace, especially in the area of alternatives to injected macromolecules, as drug formulations seek to cash in on the €6.2B worldwide market for genetically engineered protein and peptide drugs and other biological therapeutics.
Drug Delivery Carriers
Colloidal drug carrier systems such as micellar solutions, vesicle and liquid crystal dispersions, as well as nanoparticle dispersions consisting of small particles of 10–400 nm diameter show great promise as drug delivery systems. When developing these formulations, the goal is to obtain systems with optimized drug loading and release properties, long shelf-life and low toxicity. The incorporated drug participates in the microstructure of the system, and may even influence it due to molecular interactions, especially if the drug possesses amphiphilic and/or mesogenic properties.
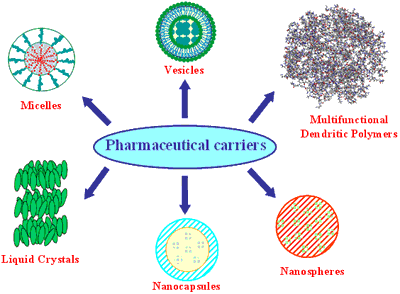 Figure 1. Pharmaceutical carriers
Figure 1. Pharmaceutical carriers
Micelles formed by self-assembly of amphiphilic block copolymers (5-50 nm) in aqueous solutions are of great interest for drug delivery applications. The drugs can be physically entrapped in the core of block copolymer micelles and transported at concentrations that can exceed their intrinsic water- solubility. Moreover, the hydrophilic blocks can form hydrogen bonds with the aqueous surroundings and form a tight shell around the micellar core. As a result, the contents of the hydrophobic core are effectively protected against hydrolysis and enzymatic degradation. In addition, the corona may prevent recognition by the reticuloendothelial system and therefore preliminary elimination of the micelles from the bloodstream. A final feature that makes amphiphilic block copolymers attractive for drug delivery applications is the fact that their chemical composition, total molecular weight and block length ratios can be easily changed, which allows control of the size and morphology of the micelles. Functionalization of block copolymers with crosslinkable groups can increase the stability of the corresponding micelles and improve their temporal control. Substitution of block copolymer micelles with specific ligands is a very promising strategy to a broader range of sites of activity with a much higher selectivity.
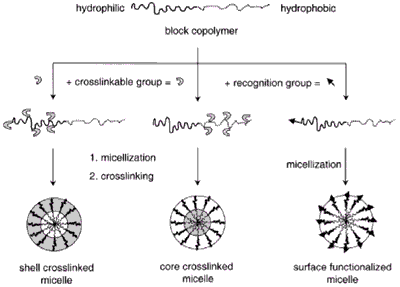 Figure 2. Block copolymer micelles.
Figure 2. Block copolymer micelles.
Liposomes are a form of vesicles that consist either of many, few or just one phospholipid bilayers. The polar character of the liposomal core enables polar drug molecules to be encapsulated. Amphiphilic and lipophilic molecules are solubilized within the phospholipid bilayer according to their affinity towards the phospholipids. Participation of nonionic surfactants instead of phospholipids in the bilayer formation results in niosomes. Channel proteins can be incorporated without loss of their activity within the hydrophobic domain of vesicle membranes, acting as a size-selective filter, only allowing passive diffusion of small solutes such as ions, nutrients and antibiotics. Thus, drugs that are encapsulated in a nanocage-functionalized with channel proteins are effectively protected from premature degradation by proteolytic enzymes. The drug molecule, however, is able to diffuse through the channel, driven by the concentration difference between the interior and the exterior of the nanocage.
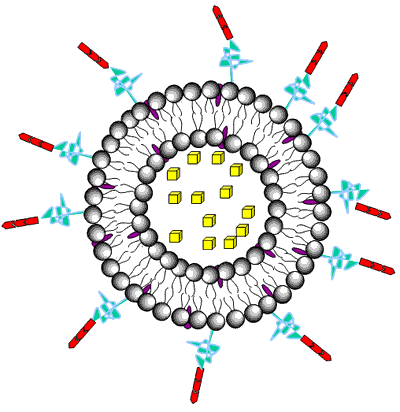 Figure 3. Drug encapsulation in liposomes.
Figure 3. Drug encapsulation in liposomes.
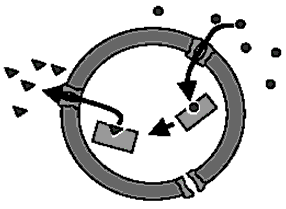 Figure 4. A polymer-stabilized nanoreactor with the encapsulated enzyme.
Figure 4. A polymer-stabilized nanoreactor with the encapsulated enzyme.
Dendrimers are nanometer-sized, highly branched and monodisperse macromolecules with symmetrical architecture. They consist of a central core, branching units and terminal functional groups. The core together with the internal units, determine the environment of the nanocavities and consequently their solubilizing properties, whereas the external groups the solubility and chemical behaviour of these polymers. Targeting effectiveness is affected by attaching targeting ligands at the external surface of dendrimers, while their stability and protection from the Mononuclear Phagocyte System (MPS) is being achieved by functionalization of the dendrimers with polyethylene glycol chains (PEG).
Liquid Crystals combine the properties of both liquid and solid states. They can be made to form different geometries, with alternative polar and non-polar layers (i.e., a lamellar phase) where aqueous drug solutions can be included.
Nanoparticles (including nanospheres and nanocapsules of size 10-200 nm) are in the solid state and are either amorphous or crystalline. They are able to adsorb and/or encapsulate a drug, thus protecting it against chemical and enzymatic degradation. Nanocapsules are vesicular systems in which the drug is confined to a cavity surrounded by a unique polymer membrane, while nanospheres are matrix systems in which the drug is physically and uniformly dispersed. Nanoparticles as drug carriers can be formed from both biodegradable polymers and non-biodegradable polymers. In recent years, biodegradable polymeric nanoparticles have attracted considerable attention as potential drug delivery devices in view of their applications in the controlled release of drugs, in targeting particular organs / tissues, as carriers of DNA in gene therapy, and in their ability to deliver proteins, peptides and genes through the peroral route.
Hydrogels are three-dimensional, hydrophilic, polymeric networks capable of imbibing large amounts of water or biological fluids. The networks are composed of homopolymers or copolymers, and are insoluble due to the presence of chemical crosslinks (tie-points, junctions), or physical crosslinks, such as entanglements or crystallites. Hydrogels exhibit a thermodynamic compatibility with water, which allows them to swell in aqueous media. They are used to regulate drug release in reservoir-based, controlled release systems or as carriers in swellable and swelling-controlled release devices. On the forefront of controlled drug delivery, hydrogels as enviro-intelligent and stimuli-sensitive gel systems modulate release in response to pH, temperature, ionic strength, electric field, or specific analyte concentration differences. In these systems, release can be designed to occur within specific areas of the body (e.g., within a certain pH of the digestive tract) or also via specific sites (adhesive or cell-receptor specific gels via tethered chains from the hydrogel surface). Hydrogels as drug delivery systems can be very promising materials if combined with the technique of molecular imprinting.
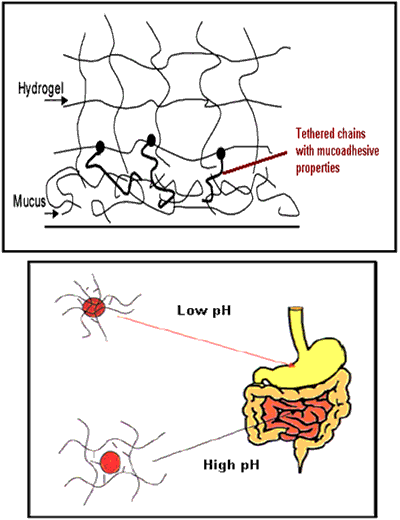 Figure 5. Pegylated and pH sensitive micro- or nanogels.
Figure 5. Pegylated and pH sensitive micro- or nanogels.
The molecular imprinting technology has an enormous potential for creating satisfactory drug dosage forms. Molecular imprinting involves forming a pre-polymerization complex between the template molecule and functional monomers or functional oligomers (or polymers) with specific chemical structures designed to interact with the template either by covalent, non-covalent chemistry (self-assembly) or both. Once the pre-polymerization complex is formed, the polymerization reaction occurs in the presence of a cross-linking monomer and an appropriate solvent, which controls the overall polymer morphology and macroporous structure. Once the template is removed, the product is a heteropolymer matrix with specific recognition elements for the template molecule.
Examples of MIP-based drug delivery systems involve: (i) rate-programmed drug delivery, where drug diffusion from the system has to follow a specific rate profile, (ii) activation-modulated drug delivery, where the release is activated by some physical, chemical or biochemical processes and (iii) feedback-regulated drug delivery, where the rate of drug release is regulated by the concentration of a triggering agent, such as a biochemical substance, the concentration of which is dependent on the drug concentration in the body. Despite the already developed interesting applications of MIPs, the incorporation of the molecular imprinting approach for the development of DDS is just at its incipient stage. Nevertheless, it can be foreseen that, in the next few years, significant progress will occur in this field, taking advantage of the improvements of this technology in other areas. Among the evolution lines that should contribute more to enhance the applicability of imprinting for drug delivery, the application of predictive tools for a rational design of imprinted systems and the development of molecular imprinting in water may be highlighted.
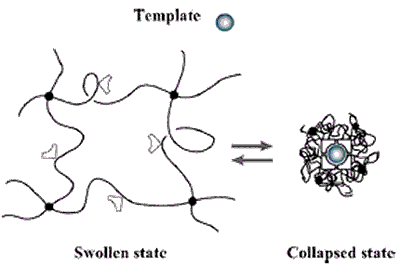 Figure 6. The volume phase transition of the hydrogel -induced by an external stimuli (e.g., a change in pH, temperature or electrical field) modifies the relative distance of the functional groups inside the imprinted cavities. This alters their affinity for the template.
Figure 6. The volume phase transition of the hydrogel -induced by an external stimuli (e.g., a change in pH, temperature or electrical field) modifies the relative distance of the functional groups inside the imprinted cavities. This alters their affinity for the template.
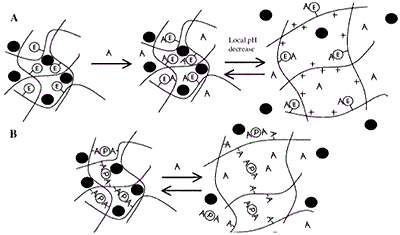 Figure 7. (A) Induced Swelling - As analyte (A) binds, the enzymatic reaction (E denotes covalently attached enzyme) produces a local pH decrease. For the cationic hydrogel, which is weakly basic, the result is ionization, swelling, and release of drug, peptide, or protein (filled circle). When A decreases in the bulk concentration, the gel shrinks. (B) Loss of Effective Cross-links - Analyte competes for binding positions with the protein (P). As free analyte binds to the protein, effective cross-links are reversibly lost and release occurs.
Figure 7. (A) Induced Swelling - As analyte (A) binds, the enzymatic reaction (E denotes covalently attached enzyme) produces a local pH decrease. For the cationic hydrogel, which is weakly basic, the result is ionization, swelling, and release of drug, peptide, or protein (filled circle). When A decreases in the bulk concentration, the gel shrinks. (B) Loss of Effective Cross-links - Analyte competes for binding positions with the protein (P). As free analyte binds to the protein, effective cross-links are reversibly lost and release occurs.
Conjugation of biological (peptides/proteins) and synthetic polymers is an efficient means to improve control over nanoscale structure formation of synthetic polymeric materials that can be used as drug delivery systems. Conjugation of suitable biocompatible polymers to bioactive peptides or proteins can reduce toxicity, prevent immunogenic or antigenic side reactions, enhance blood circulation times and improve solubility. Modification of synthetic polymers or polymer therapeutics with suitable oligopeptide sequences, on the other hand, can prevent random distribution of drugs throughout a patient’s body and allow active targeting. Functionalization of synthetic polymers or polymer surfaces with peptide sequences derived from extracellular matrix proteins is an efficient way to mediate cell adhesion. The ability of cationic peptide sequences to complex and condense DNA and oligonucleotides offers prospects for the development of non-viral vectors for gene-delivery based on synthetic polymeric hybrid materials.
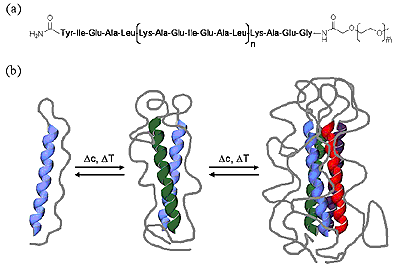 Figure 8. Bioconjugates.
Figure 8. Bioconjugates.
The field of in-situ forming implants has grown exponentially in recent years. Liquid formulations generating a (semi-)solid depot after subcutaneous injection, also designated as implants, are an attractive delivery system for parenteral application because, they are less invasive and painful compared to implants. Localized or systemic drug delivery can be achieved for prolonged periods of time, typically ranging from one to several months. Generally, parenteral depot systems could minimize side effects by achieving constant, ‘infusion-like’ plasma-level time profiles, especially important for proteins with narrow therapeutic indices. From a manufacturing point of view, in-situ forming depot systems offer the advantage of being relatively simple to manufacture from polymers. Injectable in-situ forming implants are classified into four categories, according to their mechanism of depot formation: (i) thermoplastic pastes, (ii) in-situ cross-linked polymer systems, (iii) in-situ polymer precipitation, and (iv) thermally induced gelling systems.
The ultimate goal in controlled release is the development of a microfabricated device with the ability to store and release multiple chemical substances on demand. Recent advances in microelectro-mechanical systems (MEMS) have provided a unique opportunity to fabricate miniature biomedical devices for a variety of applications ranging from implantable drug delivery systems to lab-on-a-chip devices. The controlled release microchip has the following advantages: (i) multiple chemicals in any form (e.g., solid, liquid or gel) can be stored inside and released from the microchip, (ii) the release of chemicals is initiated by the disintegration of the barrier membrane via the application of an electric potential, (iii) a variety of highly potent drugs can potentially be delivered accurately and in a safe manner, (iv) complex release patterns (e.g., simultaneous constant and pulsatile release) can be achieved, (v) the microchip can be made small enough to make local chemical delivery possible thus achieving high concentrations of drug at the site where it is needed while keeping the systemic concentration of the drug at a low level and (vi) water penetration into the reservoirs is avoided by the barrier membrane and thus the stability of protein-based drugs with limited shelf-life is enhanced.
Administration Routes
The choice of a delivery route is driven by patient acceptability, the properties of the drug (such as its solubility), access to a disease location, or effectiveness in dealing with the specific disease. The most important drug delivery route is the peroral route. An increasing number of drugs are protein- and peptide-based. They offer the greatest potential for more effective therapeutics, but they do not easily cross mucosal surfaces and biological membranes; they are easily denatured or degraded, prone to rapid clearance in the liver and other body tissues and require precise dosing. At present, protein drugs are usually administered by injection, but this route is less pleasant and also poses problems of oscillating blood drug concentrations. So, despite the barriers to successful drug delivery that exist in the gastrointestinal tract (i.e., acid-induced hydrolysis in the stomach, enzymatic degradation throughout the gastrointestinal tract by several proteolytic enzymes, bacterial fermentation in the colon), the peroral route is still the most intensively investigated as it offers advantages of convenience and cheapness of administration, and potential manufacturing cost savings.
Pulmonary delivery is also important and is effected in a variety of ways - via aerosols, metered dose inhaler systems (MDIs), powders (dry powder inhalers, DPIs) and solutions (nebulizers), all of which may contain nanostructures such as liposomes, micelles, nanoparticles and dendrimers. Aerosol products for pulmonary delivery comprise more than 30% of the global drug delivery market. Research into lung delivery is driven by the potential for successful protein and peptide drug delivery, and by the promise of an effective delivery mechanism for gene therapy (for example, in the treatment of cystic fibrosis), as well as the need to replace chlorofluorocarbon propellants in MDIs. Pulmonary drug delivery offers both local targeting for the treatment of respiratory diseases and increasingly appears to be a viable option for the delivery of drugs systemically. However, the pulmonary delivery of proteins suffers by proteases in the lung, which reduce the overall bioavailability, and by the barrier between capillary blood and alveolar air (air-blood barrier).
Transdermal drug delivery avoids problems such as gastrointestinal irritation, metabolism, variations in delivery rates and interference due to the presence of food. It is also suitable for unconscious patients. The technique is generally non-invasive and aesthetically acceptable, and can be used to provide local delivery over several days. Limitations include slow penetration rates, lack of dosage flexibility and / or precision, and a restriction to relatively low dosage drugs.
Parenteral routes (intravenous, intramuscular, subcutaneous) are very important. The only nanosystems presently in the market (liposomes) are administered intravenously. Nanoscale drug carriers have a great potential for improving the delivery of drugs through nasal and sublingual routes, both of which avoid first-pass metabolism; and for difficult-access ocular, brain and intra-articular cavities. For example, it has been possible to deliver peptides and vaccines systemically, using the nasal route, thanks to the association of the active drug macromolecules with nanoparticles. In addition, there is the possibility of improving the occular bioavailability of drugs if administered in a colloidal drug carrier.
Trans-tissue and local delivery systems require to be tightly fixed to resected tissues during surgery. The aim is to produce an elevated pharmacological effect, while minimizing systemic, administration-associated toxicity. Trans-tissue systems include: drug-loaded gelatinous gels, which are formed in-situ and adhere to resected tissues, releasing drugs, proteins or gene-encoding adenoviruses; antibody-fixed gelatinous gels (cytokine barrier) that form a barrier, which, on a target tissue could prevent the permeation of cytokines into that tissue; cell-based delivery, which involves a gene-transduced oral mucosal epithelial cell (OMEC)-implanted sheet; device-directed delivery - a rechargeable drug infusion device that can be attached to the resected site.
Gene delivery is a challenging task in the treatment of genetic disorders. In the case of gene delivery, the plasmid DNA has to be introduced into the target cells, which should get transcribed and the genetic information should ultimately be translated into the corresponding protein. To achieve this goal, a number of hurdles are to be overcome by the gene delivery system. Transfection is affected by: (a) targeting the delivery system to the target cell, (b) transport through the cell membrane, (c) uptake and degradation in the endolysosomes and (d) intracellular trafficking of plasmid DNA to the nucleus.
Future Opportunities and Challenges
Nanoparticles and nanoformulations have already been applied as drug delivery systems with great success; and nanoparticulate drug delivery systems have still greater potential for many applications, including anti-tumour therapy, gene therapy, AIDS therapy, radiotherapy, in the delivery of proteins, antibiotics, virostatics, vaccines and as vesicles to pass the blood-brain barrier.
Nanoparticles provide massive advantages regarding drug targeting, delivery and release and, with their additional potential to combine diagnosis and therapy, emerge as one of the major tools in nanomedicine. The main goals are to improve their stability in the biological environment, to mediate the bio-distribution of active compounds, improve drug loading, targeting, transport, release, and interaction with biological barriers. The cytotoxicity of nanoparticles or their degradation products remains a major problem, and improvements in biocompatibility obviously are a main concern of future research.
There are many technological challenges to be met, in developing the following techniques:
And also in the development of:
- Nano-drug delivery systems that deliver large but highly localized quantities of drugs to specific areas to be released in controlled ways;
- Controllable release profiles, especially for sensitive drugs;
- Materials for nanoparticles that are biocompatible and biodegradable;
- Architectures / structures, such as biomimetic polymers, nanotubes;
- Technologies for self-assembly;
- Functions (active drug targeting, on-command delivery, intelligent drug release devices/ bioresponsive triggered systems, self-regulated delivery systems, systems interacting with the body, smart delivery);
- Virus-like systems for intracellular delivery;
- Nanoparticles to improve devices such as implantable devices/nanochips for nanoparticle release, or multi reservoir drug delivery-chips;
- Nanoparticles for tissue engineering; e.g. for the delivery of cytokines to control cellular growth and differentiation, and stimulate regeneration; or for coating implants with nanoparticles in biodegradable polymer layers for sustained release;
- Advanced polymeric carriers for the delivery of therapeutic peptide/proteins (biopharmaceutics),
- Combined therapy and medical imaging, for example, nanoparticles for diagnosis and manipulation during surgery (e.g. thermotherapy with magnetic particles);
- Universal formulation schemes that can be used as intravenous, intramuscular or peroral drugs
- Cell and gene targeting systems.
- User-friendly lab-on-a-chip devices for point-of-care and disease prevention and control at home.
- Devices for detecting changes in magnetic or physical properties after specific binding of ligands on paramagnetic nanoparticles that can correlate with the amount of ligand.
- Better disease markers in terms of sensitivity and specificity.
References
- Charman W.N., Chan H.-K., Finnin B.C. and Charman S.A., “Drug Delivery: A Key Factor in Realising the Full Therapeutic Potential of Drugs”, Drug Development Research, 46, 316-27, 1999.
- Santini Jr, J.T., Richards A.C., Scheidt R., Cima M.J. and Langer R., “Microchips as Controlled Drug-Delivery Devices”, Angew. Chem. Int. Ed., 39, 2396-407, 2000.
- Kopecek J., “Smart and genetically engineered biomaterials and drug delivery systems”, European Journal of Pharmaceutical Sciences, 20, 1-16, 2003.
- Torchilin V.P., “Structure and design of polymeric surfactant-based drug delivery systems”, Journal of Controlled Release, 73, 137-72, 2001.
- Muller-Goymann C.C., “Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration”, European Journal of Pharmaceutics and Biopharmaceutics, 58, 343-56, 2004.
- Haag R., “Supramolecular Drug-Delivery Systems based on Polymeric Core-Shell Architectures”, Angew. Chem. Int. Ed., 43, 278-82, 2004.
- Bae Y., Fukushima S., Harada A. and Kataoka K., “Design of Environment-Sensitive Supramolecular Assemblies for Intracellular Drug Delivery: Polymeric Micelles that are Responsive to Intracellular pH Change”, Angew. Chem. Int. Ed., 42, 4640-43, 2003.
- Soppimath K.S., Aminabhavi T.M., Kulkarni A.R., Rudzinski W.E., “Biodegradable polymeric nanoparticles as drug delivery devices”, Journal of Controlled Release, 70, 1-20, 2001.
- Packhaeuser C.B., Schnieders J., Oster C.G., Kissel T., “In situ forming parenteral drug delivery systems: an overview”, European Journal of Pharmaceutics and Biopharmaceutics, 58, 445-55, 2004.
- Agnihotri S.A., Mallikarjuna N.N., Aminabhavi T.M., “Recent advances on chitosan-based micro- and nanoparticles in drug delivery”, Journal of Controlled Release, 100, 5-28, 2004.
- Sood A. and Panchagnula R., “Peroral Route: An Opportunity for Protein and Peptide Drug Delivery”, Chemical Reviews, 101, 3275-303, 2000.
- Niculescu-Duvaz I., Springer C.J., “Andibody-directed enzyme prodrug therapy (ADEPT): a review”, Advanced Drug Delivery Reviews, 26, 151-72, 1997.
- Manabe T., Okino H., Maeyama R., Mizumoto K., Nagai E., Tanaka M., Matsuda T., “Novel strategic therapeutic approaches for prevention of local recurrence of pancreatic cancer after resection: trans-tissue, sustained local drug-delivery systems”, Journal of Controlled Release, 100, 317-30, 2004.
- Ziaie B., Baldi A., Lei M., Gu Y., Siegel R.A., “Hard and Soft Micromachining for BioMEMS: Review of Techniques and Examples of Applications in Microfluidics and Drug Delivery”, Advanced Drug Delivery Reviews, 56, 145-72, 2004.
- Byrne M. E., Park K., Peppas N., “Molecular imprinting within hydrogels”, Advanced Drug Delivery Reviews, 54, 149-61, 2002.
- Vandermeulen G. W. M., Klok H-A., “Peptide/Protein Hybrid Material: Enhanced Control of Sructure nad Improved Performance through Conjugation of Biological and Synthetic Polymers”, Macromolecular Bioscience, 4, 383-98, 2003.
- Rosler A., Vandermeulen G. W. M., Klok H.-A., “Advanced drug delivery devices via self-assemply of amphiphilic block copolymers”, Advanced Drug Delivery Reviews, 53, 95-108, 2001.
- Alvarez-Lorenzo C., Concheiro A., “Molecular imprinted polymers for drug delivery”, Journal of Chromatography B, 804, 231-45, 2004.
- Vasir J. K., Tambwekar K., Garg S., “Bioadhesive microspheres as a controlled drug delivery system”, International Journal of Pharmaceutics, 255, 13-32, 2003.
- Winterhalter M., Hilty C., Bezrukov S. M., Nardin C., Meier W., Fournier D., “Controlling membrane permeability with bacterial porins: applications to encapsulated enzymes”, Talanta, 55, 965-71, 2001.