We take a closer look at the fusion of nanotechnology and CAR-T therapy through our interview with Dr. Roey Elnathan about a new approach that harnesses the capabilities of nanoneedles to efficiently deliver genetic materials to target cells. This new method could help to catapult CAR-T therapy into a new era of targeted, efficient, and adaptable cancer treatment.
Please could you introduce yourself and your current research activities?
My name is Dr. Roey Elnathan and I received my Ph.D. in Chemistry from the Centre for Nanoscience and Nanotechnology and the School of Chemistry at Tel Aviv University in 2012. I was a Research Fellow at the University of South Australia (2012–2015) and a Foundation Fellow in Nanobiotechnology (2015–2017). In 2017, I moved to Monash University in Melbourne and won the Australian Research Council DECRA Fellowship. In 2022, I moved to Deakin University (Melbourne) and won the Australian Research Council Future Fellowship.
How did you become involved in cancer research?
My research activities have evolved toward cancer—CAR T cellular immunotherapy—as a result of my ARC DECRA proposal (2016) to use cellular nanoinjection to enable the generation of functional CAR-T cells.
Image Credit: Design_Cells/Shutterstock.com
What is CAR-T cell therapy and why has it risen to prominence in cancer therapeutics?
The principle at the foundation of CAR T therapy is to couple the potency of a T cell with the specificity of an antibody to kill diseased cells.
Re-engineering patients’ immune cells to treat their cancers is a rapidly growing field of cancer treatment. One of the world’s newest forms of cancer immunotherapies, CAR-T cell therapy, has generated enormous global excitement due to promising results in treating hematologic cancers (leukaemia, lymphoma, and myeloma).
The power of personalized cellular immunotherapy lies in specific targeting and substantial clinical benefits. The enormous clinical success has energized the field to apply CAR T therapy beyond oncology, treating a broader spectrum of conditions. As such, the manufacturing of CAR T cells is rapidly evolving, incorporating emerging technologies. Among recent developments is the use of cellular nanoinjection technology.
Why are nanotechnology-enabled technologies so exciting for treatment options like CAR-T cell therapy?
The research is developing a breakthrough technology to solve a long-standing challenge in biomedicine: how to inject genetic materials into cell interiors with much greater precision but without damaging the cells’ intricate structure.
In particular, cellular nanoinjection uses programmable electroactive nanoneedles (NNs)—powerful arrays of vertical high-aspect-ratio nanostructures—allowing to achieve an intracellular delivery, with broader cargo and cell selection, improved biocompatibility and throughput, reduced safety concerns, and more uniform delivery efficacy across the targeted cellular system.
Our team develops tiny (nano) needles of specific dimensions to deliver genetic materials such as DNA into cells, giving them powerful new properties – including such functions as attacking specific cancer cells. These advances will have major potential as a platform for novel cell-based therapies for conditions that until now have eluded medical science or for which current therapies are too slow and costly to be viable.
Such a fundamental advance would open new ways of manipulating cells outside the body, creating intellectual property that would be highly attractive to Australian and international companies.
Could you discuss the results of your latest research involving nanoinjection technologies?
Our work has made significant advances in using *mechanical nanoinjection (MNI) and **electroactive nanoinjection (ENI) to maximise intracellular delivery primarily in hard to-transfect human primary T cells—all of which are functional therapeutic targets for nanoinjection.
In 2023, our work harnessed the power of both—MNI and ENI— to significantly improve the intracellular delivery of chimeric antigen receptor (CAR) genes into primary mouse/human T cells—empowering these cells with new functions such as suppressing specific lymphoma cell growth in vitro.
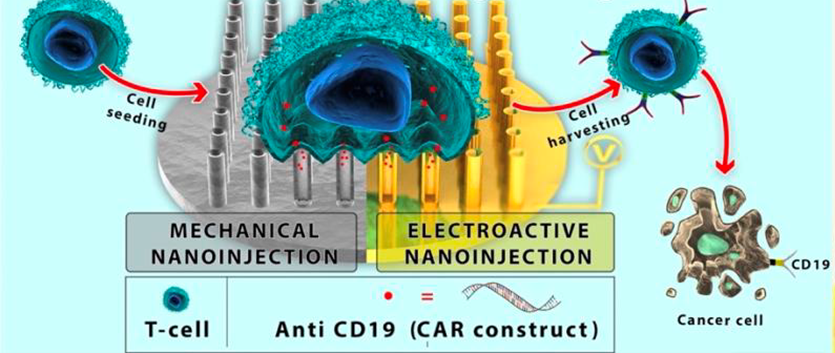
Image Credit: Roey Elnathan, Ph.D.
What is nanoinjection technology and how does it work?
Cellular Nanoinjection—the process of intracellular delivery using nanoneedles (NNs)—is a physical delivery route that efficiently negotiates the plasma membrane (PM) of many cell types (including primary immune, neural, and stem cells. This process occurs with minimal perturbation, invasiveness and toxicity, and can do so with high efficiency and throughput at high spatial and temporal resolutions.
Diverse, tuneable, nanoinjection platforms are now providing major advantages in the precise manipulation of increasingly complex cellular processes—such as intracellular delivery/sampling, intracellular probing of an action potential, immunomodulation, and biosensing.
Did the team encounter any challenges during the investigation?
We encountered many challenges. To elaborate, the cytoplasm of a living cell is a crowded, dynamic place, where intracellular access and drug transport are tightly constrained to ensure healthy cell function and behaviour. Conventional approaches to accessing the cell interior for delivery and sensing are powerful, but typically perturb cell function significantly. The genius of nanoneedle technology is their capacity to give access to the cell with minimal disruption, paving the way for the optimal intracellular delivery of advanced therapeutics. The bonus is that nanoneedles give access to cells rapidly, efficiently, and at low cost, for most of the important types of cells and tissues.
What impact might this nanoneedle-based treatment have on the availability and efficacy of CAR-T cell therapy treatment?
The technology has only been proved in killing cancer cells in vitro; we now assess the anti-tumour efficacy of NN-engineered CAR-T cells in vivo via a preclinical human xenograft model.
But manufacturing today’s viral-based CAR-T cell therapy is extremely expensive (manufacturing costs > $0.5M), involving complex (a lengthy viral transfection process with many biosafety checkpoints due to an elevated risk of insertional mutagenesis) and slow manufacturing processes (global limitation in the capacity to produce clinical grade vector).
These are serious hurdles for widespread implementation of CAR-T cell therapy—a problem that will worsen as demand increases. If only CAR-T cell therapy were able to offer rapid, safe, and non-viral CAR-T cell production, it would unlock the therapy's full potential, moving it from a boutique to a widely affordable solution.
The number of nanoneedle patents increases year on year, showing that the nanoneedle landscape is still very fertile for the germination of more advanced ideas and the value of these ideas seems to be growing.
The target markets for these technologies are all comparatively young and still very open to disruptive innovation that improves efficiency and or reduces costs. Yet, the value of these markets and their projected growth is significant, indicating a strong promise for long-term sustainability if the technology can establish itself.
Are you hopeful that there will be a future where expensive treatments like CAR-T are widely available? What more must be done for this to occur?
Concurrently with the ongoing innovation to develop new platforms for CAR T cells, substantial effort is already underway to optimize the ex vivo CAR T cell production process. Much will be learned in this decade as more CAR-T cell products are used to treat solid cancers (rather than blood cancer). We are only beginning to realize the full potential of this living drug (i.e CAR-T cells as a live drug).
Now that this research has been published, what next steps must be taken to take it to clinical trial? What is the estimated timeline for this?
The technology has only been proved in killing cancer cells in vitro; we now assess the anti-tumor efficacy of NN-engineered CAR-T cells in vivo via a preclinical human xenograft model. I think that in 2-3 year’s time (if all goes according to the plan), we could be directing the technology toward clinical trial.
*Electroactive nanoinjection (ENI)—mediated by coupling nanoneedles with an electric field—is a new route for activating electroporation at the nanoscale, by inducing a highly localised and uniform electric field; this creates ‘holes’ through the cell membrane at the NN–cell point of contact.
**Mechanical nanoinjection (MNI)—mediated by the application of centrifugal force—significantly improving our control and reliability of delivery by increasing membrane permeability.
About Roey Elnathan, Ph.D.
 Working across the boundaries of disciplines from fundamental material science to cellular immunotherapy (CAR-T cell) technology, together with Australian Laureate Professor Voelcker (Monash), Roey Elnathan has led an intensely interdisciplinary project team—clinical CAR-T cell researchers, industry researchers from ULVAC Inc Japan, and researchers from Deakin, Monash, University of Adelaide, and Westmead Institute of Medical Research.
Working across the boundaries of disciplines from fundamental material science to cellular immunotherapy (CAR-T cell) technology, together with Australian Laureate Professor Voelcker (Monash), Roey Elnathan has led an intensely interdisciplinary project team—clinical CAR-T cell researchers, industry researchers from ULVAC Inc Japan, and researchers from Deakin, Monash, University of Adelaide, and Westmead Institute of Medical Research.
The advances have had considerable impact on the nascent field of cell–material interfaces, by unlocking new ways of manipulating cells outside the body, with precise spatiotemporal resolution and minimal perturbation.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.