Researchers at the U.S. Department of Energy's Argonne National Laboratory have developed a systematic method to improve the stability of antibodies. The technique could lead to better biosensors, disease therapeutics and diagnostic reagents and non-laboratory applications, including environmental remediation.
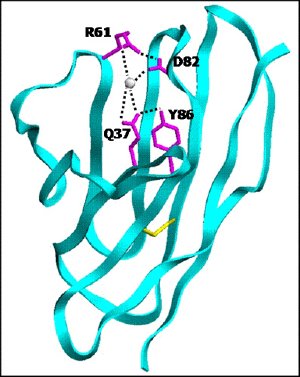 Protein stability arises from networks of inter-atomic interaction. In this protein, a network is formed when Q37, a surface amino acid residue, forms a hydrogen bond with amino acid residue Y86 and interacts with amino acid residue D82 through a bridging water molecule. Image courtesy Raj Pokkuluri.
Protein stability arises from networks of inter-atomic interaction. In this protein, a network is formed when Q37, a surface amino acid residue, forms a hydrogen bond with amino acid residue Y86 and interacts with amino acid residue D82 through a bridging water molecule. Image courtesy Raj Pokkuluri.
Antibodies are proteins produced by humans and animals to defend against infections; they are also used to diagnose and treat some diseases and detect toxins and pathogens. "The primary issue with antibodies is that they are fragile and short-lived outside of cooler temperature-controlled environments, making their usefulness usually limited to laboratory applications," said Argonne senior biophysicist Fred Stevens, the project's principle investigator.
Specifically, "stabilized antibodies, with full functionality, could be used in diagnostic and detection kits that can survive in less than optimal environments and be stockpiled for years at a time," Stevens said. "They could be used to combat diseases like cancer. They can also be used as the basis for biosensors that can continuously detect for pathogens like botulinum, ricin and anthrax in places such as airports and subway stations—locations where it is not currently possible to provide ongoing detection of pathogens because antibodies cannot tolerate the environmental conditions."
Argonne has provided funding toward Stevens’ research. Earlier research funded by the National Institutes of Health showed that it was possible to stabilize antibodies after a team led by Stevens unexpectedly discovered that natural antibodies contain stabilizing amino acid replacements.
Antibodies are made up of four polypeptides—two light chains and two heavy chains. These chains are made up of modules known as constant and variable domains. The light and heavy chain each have a variable domain, which come together to form the antigen binding site. Because of the great diversity of amino acids in the variable domains, different antibodies are capable of interacting with an effectively unlimited number of targets.
Sometimes this variability comes at a price; the amyloid-forming light chains were less stable than their normal counterparts. However, even amyloid-forming light chains have amino acid substitutions that improve stability. When seven of these amino acid changes were introduced into an amyloid-forming variable domain, a billion-fold improvement in thermodynamic stability was obtained reflecting a much higher ratio of native protein folds to unfolded proteins—a major determinate of antibody shelf life.
"Our work at this detailed level had taught us that antibody stabilization is possible, but we needed to find out if antibodies could be stabilized without compromising their function and do so with moderate experimental investment," Stevens said. Recent work suggests these goals are potentially achievable. To proactively improve the stability of a different antibody variable domain, Argonne researchers drew up a short list of 11 candidate amino acid changes. Four of the amino acid changes improved antibody stability and when combined together in the original domain, they provided a 2,000-fold improvement in stability.
A follow up experiment using a functional antibody fragment was able to improve antibody stability comparably, with no loss of antibody functionality. Both experiments required approximately one month to accomplish instead of the potentially open-ended time required for most protein stabilization projects.
There is a correlation between thermodynamic stability and thermal stability; the billion-fold improvement in thermodynamic stability increased the thermal resistance of the protein to heating, resulting in a “melting temperature” of about 160 degrees Fahrenheit. "However, still unanswered is whether it is possible to be confident about improving the stability of any antibody generated against a particular target," Stevens said. "Our research indicates that stabilization of antibodies is possible. We project that it could be possible to generate the data to guide stabilization of every future antibody in the near future."
Argonne’s Office of Technology Transfer is actively seeking participation from industry for licensing as well as funding for further development of this technology.