May 5 2016
A new fabrication method to make polymer membranes with improved permeance using molecular design has been developed by researchers from Imperial College London.
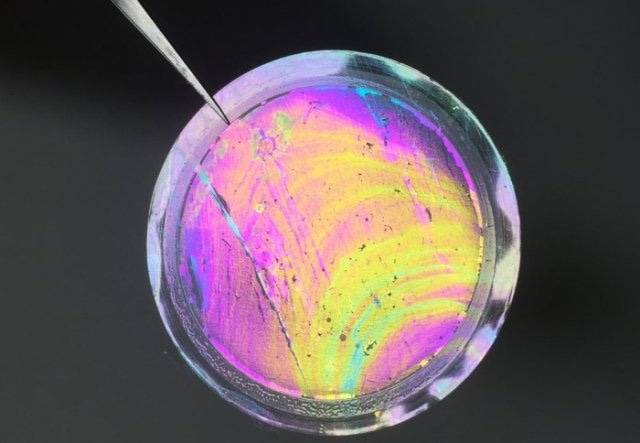 This is a photo of a free-standing polymer nanofilm membrane (200 nm in thickness) with enhanced microporosity, supported on porous alumina. (credit: Qilei Song, Department of Chemical Engineering, Imperial College London.)
This is a photo of a free-standing polymer nanofilm membrane (200 nm in thickness) with enhanced microporosity, supported on porous alumina. (credit: Qilei Song, Department of Chemical Engineering, Imperial College London.)
The molecularly designed polymer membranes produced by the novel synthetic technique can improve the efficiency of chemical separation processes by up to two orders of magnitude when compared to traditional membranes.
Chemical separation processes are generally expensive and existing methods often employ thermal separation processes like evaporation and distillation, which represent 10% to 15% of the yearly global energy consumption. A highly efficient, non-thermal option will be produced when synthetic polymer membranes are used to separate chemicals and gases, showing promise for a significant reduction in energy consumption, lowering carbon dioxide emissions, and mitigating pollution.
Traditional membranes are not able to deliver superior performance in large-scale separation processes due to their relatively low permeance of liquids and gases. It is a difficult process to develop membranes with an organic solvent resistance to use in chemical separation and petrochemical refining processes. Researchers around the world are exploring ways to develop cheaper and superior performance membranes with high stability, high permeance, and high molecular selectivity for effective separation of liquids and gases. Such high-performance microporous polymer membranes can now be produced using this new synthetic approach.
The study results have been reported in the Nature Materials journal.
The research group headed by Professor Andrew Livingston from the Department of Chemical Engineering at Imperial College London is involved in the development of new approaches for membrane separations, which have a range of industrial applications such as carbon dioxide capture, solvent nanofiltration, desalination and purification of oil and natural gas.
Using the breakthrough method, polymer membranes are produced by linking contorted monomers to create cross-linked network polymers. A new class of microporous polymers, dubbed ‘polymers of intrinsic microporosity (PIMs),’ were synthesized using these twisted monomers. PIMs possess a higher volume of internal cavities, which give the membranes a high permeance. Their network polymer ensures their rigidity and stability by serving as a scaffold.
The new approach is a combination of two techniques, namely microporous polymer synthesis and interfacial polymerization. This combination enabled the researchers to synthesize microporous polymer membranes with a controlled thickness down to 20 nm.
This work reports new methods of fabricating polymer membranes using a molecular design approach. We are able to design the free volume, which acts as pores in the membrane, by choosing the monomers used to make the membrane separating layer. So we have managed for the first time to create interconnected 3D polymer network membranes in which we can control the size of pores and their connectivity. This means we can make a more accurate separation between molecules and at a higher processing rate, making more efficient separations with less consumption of energy.
Professor Andrew Livingston, Department of Chemical Engineering, Imperial College London
"We demonstrated a simple approach to preparing microporous thin polymer membranes using the aromatic polyester chemistry as an example" said Dr. Maria F. Jimenez-Solomon, co-lead author of the study and a postdoctoral research associate from Livingston's group at Imperial College London. “However, the approach is not limited to synthesising polyesters, it has opened up new ways of synthesising membrane materials using a range of contorted molecules”.
To optimize and scale up the synthetic approach, we performed extensive characterisations to understand the structure and properties of these polymer membranes, however there are still many interesting scientific questions to study in the future. We expect that by tuning the molecular structure of the polymers in combination with nanoscale control of the membrane, the performance of polymer membranes can be enhanced even further.
Dr. Qilei Song, Junior Research Fellow, Department of Chemical Engineering, Imperial College London
Dr. Kim E. Jelfs, a co-author on the paper and a Royal Society University Research Fellow at the Department of Chemistry at Imperial College London, performed molecular simulations to corroborate the porous structures of polymer membranes;
The computational approaches allow us to elucidate the nature of the materials, for example, we can predict the polymer structure and porosity based on the large scale computational screening of precursor libraries.
Dr. Kim E. Jelfs, Royal Society University Research Fellow, Department of Chemistry, Imperial College London
In their paper, the scientists described the applications of the molecularly designed polymer membranes in organic solvent and gas separations. Going forward, the researchers will use their method to develop a variety of porous polymers for a range of industrial applications, including purification of pharmaceuticals, water desalination and purification, and separation of hydrocarbons for petrochemical applications.
If we are able to use membranes to accurately separate molecules which are in organic solvents, we can work towards replacing distillation and evaporation processes with more energy-efficient membrane separation technologies.
Professor Andrew Livingston, Department of Chemical Engineering, Imperial College London
The research work received funding from the Engineering and Physical Sciences Research Council (EPSRC), 7th Framework Programme of the European Commission's Marie Curie Initiative, Imperial College Junior Research Fellowship, and the Royal Society University Research Fellowship.