Jun 22 2016
Anaplastic thyroid cancer (ATC) is a highly life threatening form of thyroid cancer with a mortality rate of nearly 100% and an average survival time of three to five months. One promising approach to treat this type of cancer and others is RNA interference (RNAi) nanotechnology. However delivery of RNAi agents to the precise area of the tumor has been a struggle.
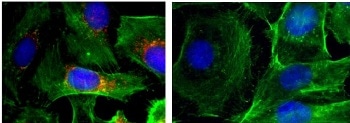 These are immunofluorescence images of cells (nuclei shown in blue; actin shown in green; BRAF shown in red). Left: control; right: after treatment with nanoparticles that silence BRAF. (Photo credit: Image courtesy of Jinjun Shi, Brigham and Women's Hospital)
These are immunofluorescence images of cells (nuclei shown in blue; actin shown in green; BRAF shown in red). Left: control; right: after treatment with nanoparticles that silence BRAF. (Photo credit: Image courtesy of Jinjun Shi, Brigham and Women's Hospital)
A team of researchers from Brigham and Women’s Hospital partnered with another team from Massachusetts General Hospital to create a unique nanoplatform that enables them to efficiently deliver the RNAi agents to the areas with cancerous tumors and suppress the growth of the tumor. The agent also could decrease metastasis in preclinical representation of ATC. The collaborative efforts of the researchers have been published in The Proceedings of the National Academy of Sciences.
We call this a ‘theranostic’ platform because it brings a therapy and a diagnostic together in one functional nanoparticle. We expect this study to pave the way for the development of theranostic platforms for image-guided RNAi delivery to advanced cancers.
Jinjun Shi, PhD, Assistant Professor of Anesthesia, Brigham and Women's Hospital
A decade ago, the innovative breakthrough discovery of RNAi won the Nobel Prize in Physiology or Medicine. This discovery has allowed researchers to restrict mutated genes, including ones that cancers depend on to thrive, live and metastasize. Several ATCs rely upon mutations in the frequently mutated cancer gene BRAF.
The team hoped to halt the progress and the spread of ATC to lungs and other organs by delivering RNAi agents directly to the target area and silence this mutated gene. When RNAi is delivered by itself, enzymes tend to break it down, or the agent gets filtered out by the kidneys even before it makes contact with tumor cells. Sometimes when RNAi agents do make contact with the tumor, they are rejected by the cancer cells or are not able to penetrate the tumor.
To beat these hurdles, the researchers used nanoparticles to deliver the RNAi agents to ATC tumors. The nanoparticles were coupled with a near-infrared fluorescent polymer, which enabled the researchers observe where the nanoparticles gathered in a mouse model of ATC.
The researchers were able to determine that the nanoparticles had indeed made contact with the ATC in the thyroid by calculating the glow from the near-infrared fluorescent polymer. They noticed that the nanoparticles circulated for a stretch of time in the blood stream and then gathered in high concentrations in the tumors.
Additionally, the team revealed proof that BRAF also could be successfully silenced at the target areas. When the cells grown in a dish were treated with the nanoparticles coupled with RNAi agents, the cell growth radically decreased and the number of cancerous cells that could move about reduced by to about 15-fold. In mouse replicas, tumor progress was reduced and there was lesser occurrences of metastases.
To be able to move this new treatment platform into clinical scenarios, the team highlights the significance of having an imaging diagnostic that will offer them a means to speedily evaluate which patient will benefit the most from RNAi nanotherapeutics.
Most patients who present to surgeons with anaplastic thyroid cancer are out of options and this new research gives these patients some options. Having an approach that allows us to rapidly visualize and simultaneously deliver a targeted therapy could be critical for the efficient treatment of this disease and other lethal cancers with a poor prognosis.
Sareh Parangi, MD, Associate Professor, Massachusetts General Hospital
This research was supported by the NIH grants R00CA160350, CA200900, CA149738, EB015419, and U54-CA151884; by the National Research Foundation of Korea K1A1A2048701; and by the DoD PCRP Synergistic Idea Development Award; by the Koch-Prostate Cancer Foundation (PCF) Program in Nanotherapeutics, Movember-PCF Challenge Award and PCF Young Investigator Award.