Nov 16 2018
Researchers at Tokyo Institute of Technology (Tokyo Tech) have successfully created subnano-sized metallic particles that can be effectively used as catalysts for oxidizing hydrocarbons. Compared to popular Au-Pd bimetallic nanocatalysts, the new catalysts can be up to 50 times more effective.
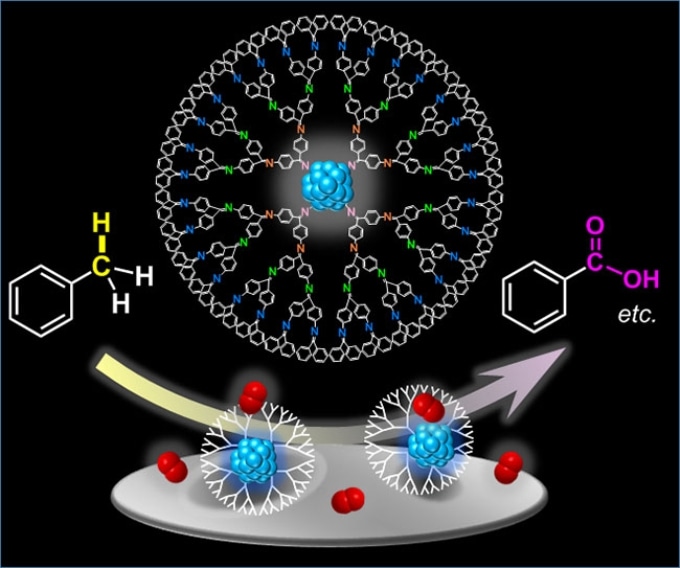 Catalytic activity of subnano-sized metallic particles within dendrimers. Each dendrimer molecule hosts a subnano-sized metallic particle that allows for the oxidation of aromatic hydrocarbons, such as toluene (left), to produce useful organic compounds, such as benzoic acid (right). Oxygen molecules are represented in red. (Image credit: Angewandte Chemie)
Catalytic activity of subnano-sized metallic particles within dendrimers. Each dendrimer molecule hosts a subnano-sized metallic particle that allows for the oxidation of aromatic hydrocarbons, such as toluene (left), to produce useful organic compounds, such as benzoic acid (right). Oxygen molecules are represented in red. (Image credit: Angewandte Chemie)
Organic compounds are used across all types of industries. To create a wide range of these useful compounds, oxidation of aromatic hydrocarbons is very important. However, oxidation processes like these require the use of solvents and catalysts, which are often environmentally dangerous. Therefore, detecting a solvent-free oxidation process through nanosized catalytic particles has attracted a great deal of interest. Composed of noble metals, sub-nanoscale catalytic particles (subnanocatalysts or SNCs) are much better at their job, thanks to their special electronic state and increased surface area that lead to favorable effects for oxidizing hydrocarbons and, at the same time, prevent them from being oxidized themselves. As a result, SNCs are economical because the amount of metal needed for SNCs is relatively lower than for nanocatalysts.
A research team including Dr Miftakhul Huda, Dr Takamasa Tsukamoto, Keigo Minamisawa, and Dr Makoto Tanabe from Tokyo Tech, headed by Professor Kimihisa Yamamoto, used dendrimers to develop multiple types of SNCs. Dendrimers are tree-like spherical molecules that can be applied as a template to contain the required catalysts.
Dendrimer is expected to provide internal nanospaces that could be suitable for catalytic conversion in the presence of metal particles.
Kimihisa Yamamoto, Professor, Tokyo Institute of Technology.
The researchers developed a wide range of catalysts of varied sizes, based on the number of atoms of each catalytic particle and the noble metal used. Next, they compared the performance of these catalysts to identify the best noble metal for fabricating SNCs and then attempted to establish the mechanism behind their increased catalytic activity. They observed that while smaller SNCs were better, less oxophilic metals like platinum were superior. The researchers hypothesized that the surface of platinum SNCs does not oxidize easily, making them reusable and leading to the highest catalytic performance of the Pt19 SNC that can be as much as 50 times more effective when compared to the standard Au-Pd nanocatalysts.
The researchers will continue to study these catalytic phenomena to gain a better understanding.
The development of a more detailed mechanism including theoretical considerations is currently in progress.
Dr Makoto Tanabe, Tokyo Institute of Technology.
The use of such catalysts may considerably help in decreasing pollution and improving the effective applications of metal resources available on Earth.