Apr 30 2019
Innovative battery technologies will be required to meet the demands of electronics in the future. In this regard, lithium-sulfur batteries may provide a solution as they have a hypothetical energy density about five times that of lithium-ion batteries.
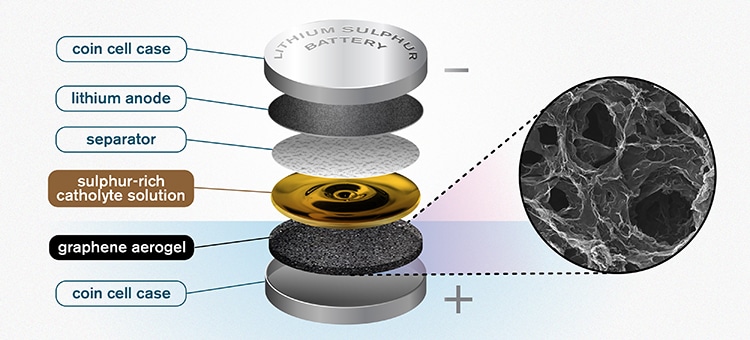 The Chalmers design for a lithium-sulfur battery. The highly porous quality of the graphene aerogel allows for high enough soaking of sulfur to make the catholyte concept worthwhile. (Image credit: Yen Strandqvist/Chalmers University of Technology)
The Chalmers design for a lithium-sulfur battery. The highly porous quality of the graphene aerogel allows for high enough soaking of sulfur to make the catholyte concept worthwhile. (Image credit: Yen Strandqvist/Chalmers University of Technology)
Now, a research team at Chalmers University of Technology, Sweden, has recently reported a potential breakthrough for this kind of battery, and they achieved this by utilizing a catholyte with the aid of a graphene sponge.
The innovative concept devised by the team is a sponge-like, porous aerogel composed of reduced graphene oxide. This aerogel functions as a free-standing electrode within the battery cell, enabling better and higher utilization of sulfur.
Normally, four parts are included in a standard battery. First, a pair of supporting electrodes are included that are covered with an active substance, and these electrodes are called a cathode and an anode. An electrolyte, usually a liquid, is present in between them and enables ions to be transferred to and fro. A separator is the fourth component that serves as a physical barrier, inhibiting contact between both the electrodes and, at the same time, enabling the transfer of ions.
Earlier, the investigators tried to integrate the electrolyte and cathode into a liquid, what is known as “catholyte.” Such a concept would not only save the battery weight but would also provide better power and faster-charging capabilities. Now, the fabrication of the novel graphene aerogel has made the concept feasible, providing some highly potential outcomes.
The scientists initially integrated a thin layer of the porous graphene aerogel into a traditional coin cell battery case.
You take the aerogel, which is a long thin tube, and then you slice it—almost like a salami. You take that slice, and compress it, to fit into the battery.
Carmen Cavallo, Study Lead Researcher, Department of Physics, Chalmers University of Technology.
Subsequently, the catholyte—a sulfur-rich solution—was added to the battery. Acting as the support, the highly porous aerogel soaks up the solution like a sponge.
The porous structure of the graphene aerogel is key. It soaks up a high amount of the catholyte, giving you high enough sulphur loading to make the catholyte concept worthwhile. This kind of semi-liquid catholyte is really essential here. It allows the sulphur to cycle back and forth without any losses. It is not lost through dissolution—because it is already dissolved into the catholyte solution.
Carmen Cavallo, Study Lead Researcher, Department of Physics, Chalmers University of Technology.
The separator is also covered with some of the catholyte solution, so that it can fulfill its electrolyte role. Such an approach also maximizes the battery’s sulfur content.
Lithium-ion batteries constitute a majority of batteries currently used in everything from electric cars to mobile phones. However, this kind of battery is reaching its limitations, and hence novel chemistries are becoming vital for applications, where higher power is needed. A number of benefits are provided by lithium-sulfur batteries, including relatively higher energy density. At present, the best commercially available lithium-ion batteries operate at approximately 300 watt-hours/ kg, with a hypothetical maximum of about 350. On the other hand, lithium-sulfur batteries have a hypothetical energy density of about 1000 to 1500 watt-hours/kg.
Furthermore, sulphur is cheap, highly abundant, and much more environmentally friendly. Lithium sulphur batteries also have the advantage of not needing to contain any environmentally harmful fluorine, as is commonly found in lithium-ion batteries.
Aleksandar Matic, Study Lead and Professor, Department of Physics, Chalmers University of Technology.
So far, the issue with lithium-sulfur batteries has been their lack of stability and thus the resulting low cycle life. Present versions of batteries tend to degenerate rapidly and have a restricted life span with an unfeasibly low number of cycles. However, while testing their latest prototype, the researchers at Chalmers University of Technology showed a capacity retention of 85% following 350 cycles.
The innovative design eliminates the two major issues associated with the degradation of lithium-sulfur batteries—first is that sulfur has a tendency to dissolve into the electrolyte and disappears, and second is a “shuttling effect,” in which sulfur molecules travel from the cathode to the anode. In the latest design, these unwanted problems can be significantly lowered.
However, the team noted that more research needs to be done before the novel technology can reach its full market potential.
Since these batteries are produced in an alternative way from most normal batteries, new manufacturing processes will need to be developed to make them commercially viable.
Aleksandar Matic, Study Lead and Professor, Department of Physics, Chalmers University of Technology.