Image Credit: Shutterstock.com/ DooDee Studio
We found that the mechanism of polymerization is the same in both cases, but the kinetics of polymerization differs significantly. The reactivity of the monomer in solution turned out to be about five times lower than in the melt, which is most likely due to the effect of solvation – the interaction of the solvent with the solute.
Andrey Galukhin, Group leader and Associate Professor, Kazan Federal University
Ilya Nikolaev, a PhD candidate, whose forthcoming thesis is committed to the dependencies between the structure of aryl cyanates and their reactivity.
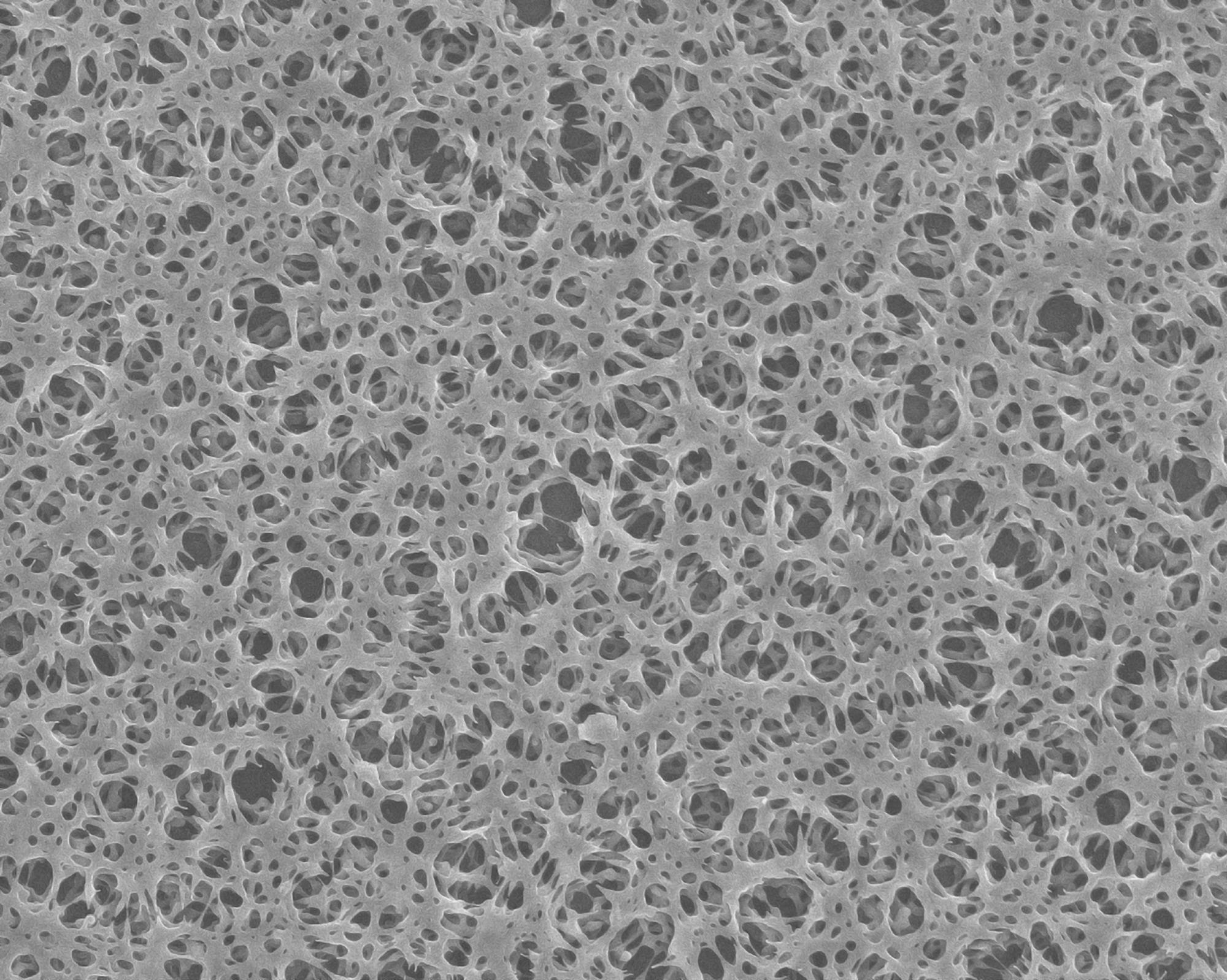
When heated, aryl cyanates enter into a polymerization reaction, the kinetics of which we are studying. In this publication, we examined the effect of a high-boiling solvent (diphenyl sulfone) on the kinetics of polymerization of the monomer we synthesized. Polymerization in a solvent medium makes it possible to obtain promising nanoporous polymer materials. Such materials can be used to store, for instance, hydrogen.
Andrey Galukhin, Group leader and Associate Professor, Kazan Federal University
Dr. Galukhin considers that one of the main technical issues associated with hydrogen energy is the storage of this very light and explosive gas. When gas molecules penetrate a porous material, a good adsorbent, they “stick” to its walls, and if the surface area of these walls is huge, then the material has the ability to “absorb” a considerable amount of gas.
Hydrogen is very light, so only very little of it fits into cylinders, implying that storing this gas by this method is inefficient. When a nanoporous material is added to a container, it tends to absorb gas like a sponge, and consequently, much more of it will fit into the storage system.
Furthermore, a new class of adsorbent is required for removing oil spills on water and for making different sensors, and new scientific outcomes can be employed to improve the conditions for achieving such materials depending on aryl cyanate monomers.
Journal Reference:
Galukhin, A., et al. (2021) Solvent-induced changes in the reactivity of tricyanate esters undergoing thermal polymerization. Polymer Chemistry. doi.org/10.1039/D1PY01088C.