Viral diseases can have significant clinical and financial impacts worldwide. The COVID-19 pandemic has resulted in severe clinical consequences, with the World Health Organization reporting over 267 million documented cases and more than 5.26 million fatalities so far.
The economic impact was also severe: damages to the global market are expected to total £8 trillion in 2020–21 and £22 trillion in the next five years. Seasonal influenza is also thought to be responsible for 250,000–500,000 fatalities per year throughout the globe.
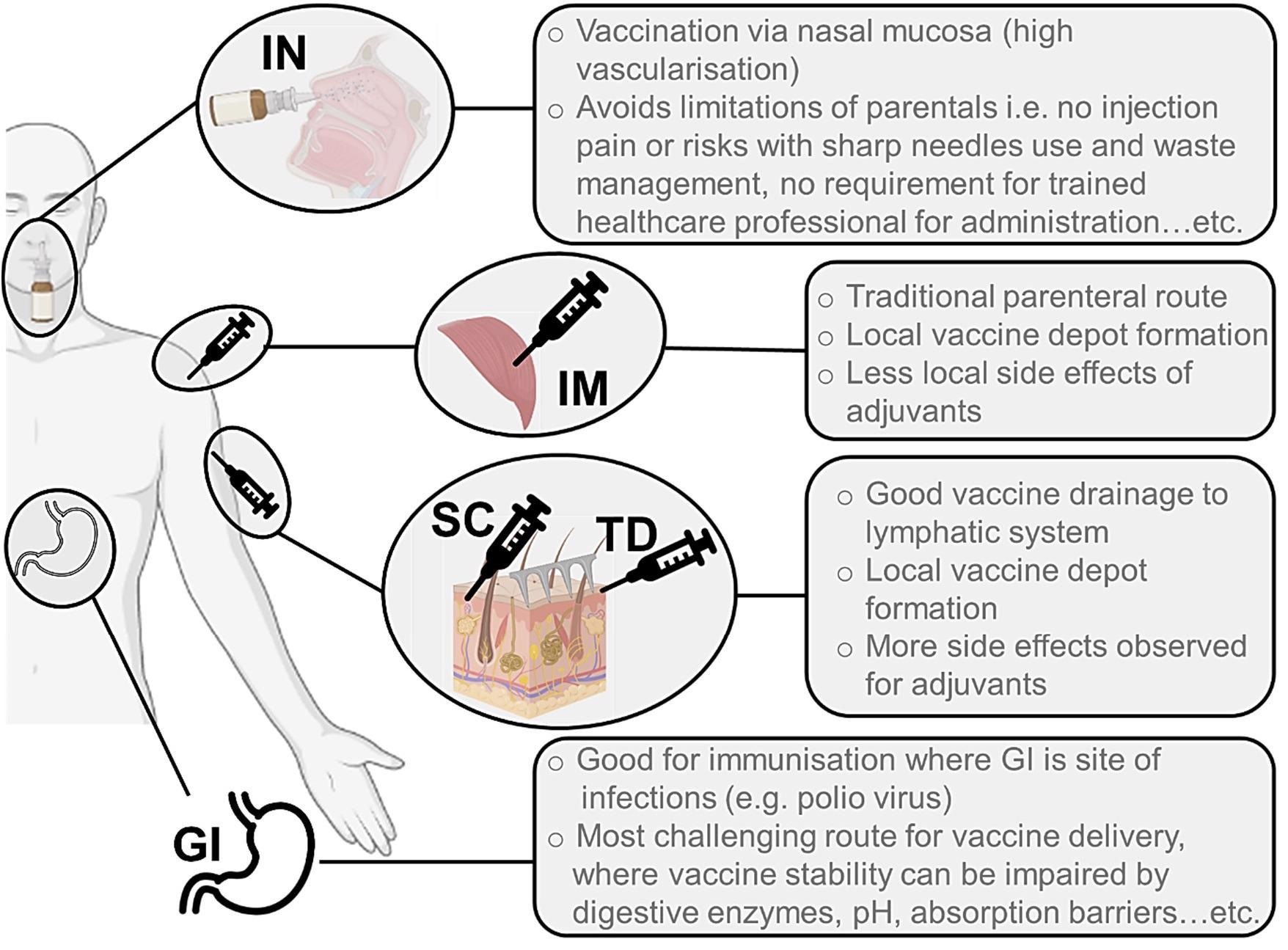
Illustration of the main routes of administration used for delivery of vaccines against viruses. These are IN (intranasal), IM (intramuscular), SC (subcutaneous), TD (transdermal) and oral (GI, gastrointestinal). Image Credit: Patil-Sen, Y. et al.
The use of vaccines is a successful approach for preventing infectious diseases.
Vaccines are classified as whole-virus, subunit, or genetic vaccines. These include a weaker version of the virus that stimulates the immune system without producing sickness.
The production of these different forms of viral vaccines has proved difficult over the decades, but it has been successful owing to rapid advancement and improvements in biotechnology.
However, significant effort has to be made to increase the effectiveness and safety of existing vaccines to achieve stronger viral control.
Challenges of Vaccine Delivery
Initially, the majority of vaccines were made of live, virulent, or inactivated viruses, which elicited an immune reaction through the same mechanisms used to combat the original illness. However, these vaccines have a risk of severe side effects, prompting the creation of synthetic antigens with improved safety profiles.
Most vaccines, despite their efficacy, have a high sensitivity to cold and are unstable at high temperatures. This means that they must be maintained and delivered in a controlled environment known as a "cold chain." Because of this need, vaccine distribution in third world countries is severely hampered, since resources for maintaining cold chain transportation are often in short supply in these areas.
The administration of vaccine antigens to important tissues such as lymph nodes (LN), which contain many immune cells, is another key difficulty in vaccine distribution.
Targeting these tissues is dependent not only on the dosage forms but also on the physical and chemical features of the vaccine delivery method.
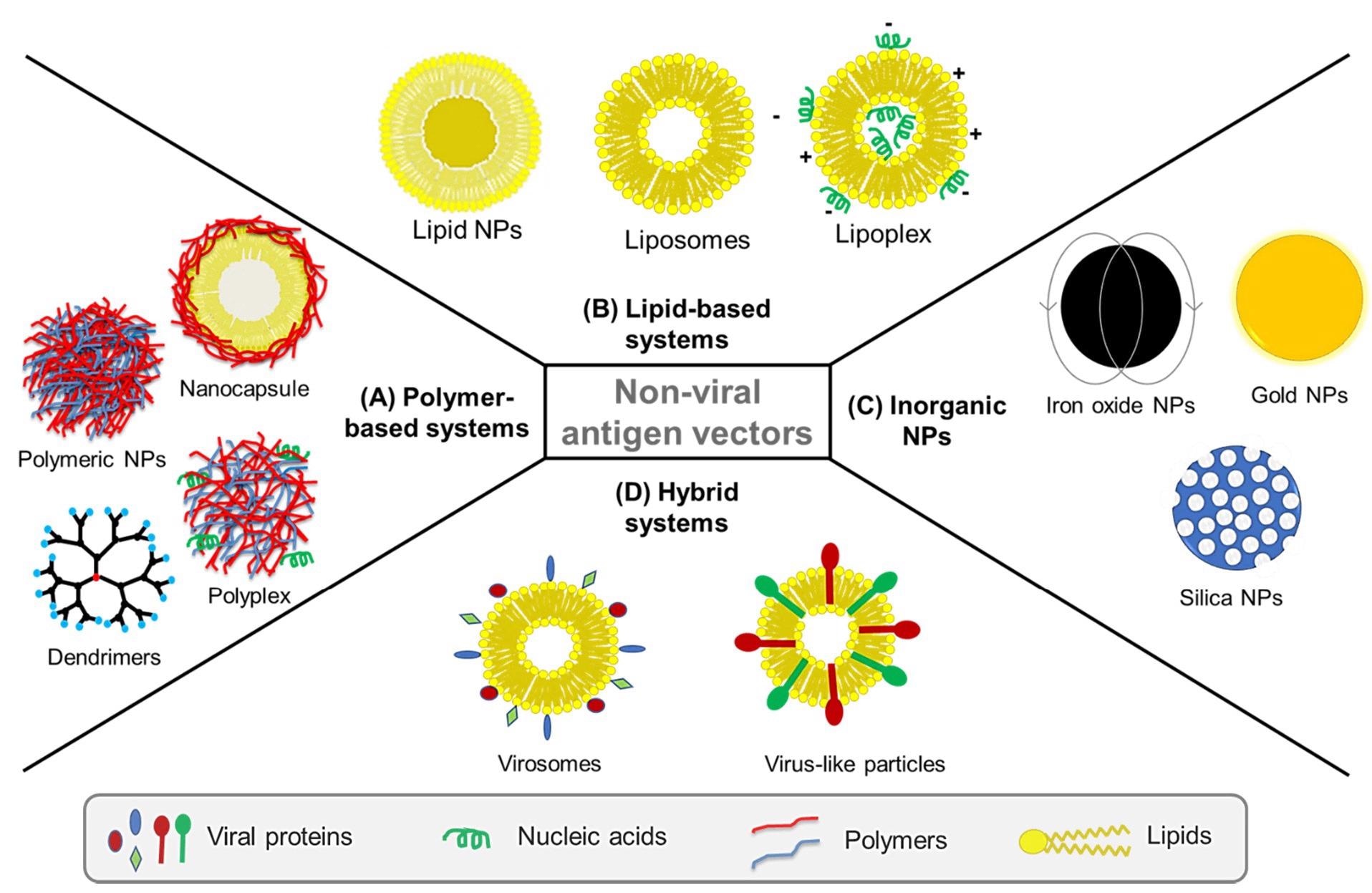
Nonviral vectors used for delivery of vaccines against viruses are classified into: (A) polymer-based systems such as polymeric nanoparticles (NPs), polyplexes, polymeric dendrimers and polymeric nanocapsules, (B) lipid-based systems such as liposomes, lipid NPs and lipoplexes, and (C) inorganic NPs such as iron oxide, gold, and mesoporous silica NPs. In addition to nonviral vectors, (D) hybrid systems such as virosomes and virus-like particles (VLPs) have been also developed to combine nonviral systems like liposomes with viral elements, for instance decorating liposomes with viral glycoproteins to imbue the system with viral immunogenicity. Image Credit: Patil-Sen, Y. et al.
Delivery Systems for Vaccines
An adequately designed vaccine delivery system is critical for increasing immunization efficacy and enhancing vaccine durability. It may also aid in the management of dosage regularity, patient comfort, and operations for mass vaccination programs, among other things.
When used in conjunction with viral vectors, it is possible to transport genetic material containing the pathogen's antigen immediately to the host cell, which, when generated, triggers an immune reaction. This takes advantage of the precision of the vectors' infection and may result in the generation of large amounts of the target protein in the bloodstream.
Liposomes, virosomes, and VLPs are examples of nanoparticle drug delivery systems that have reached the market. These mechanisms limit protein release prematurely and extend protein expression for effective viral disease immunization.
However, most nanocarriers are still in the early stages of research and clinical development. Because of their nanoparticulate size and vast surface area, these carriers are prone to coagulation, raising concerns about their toxicity and stability in clinical settings.
Hydrogels and microneedles have shown significant promise for targeted vaccination, among other advanced delivery technologies. Due to their good viscoelastic and excellent biocompatibility qualities, both polymeric and protein-based hydrogels are viable for targeted delivery of viral proteins in preliminary experiments.
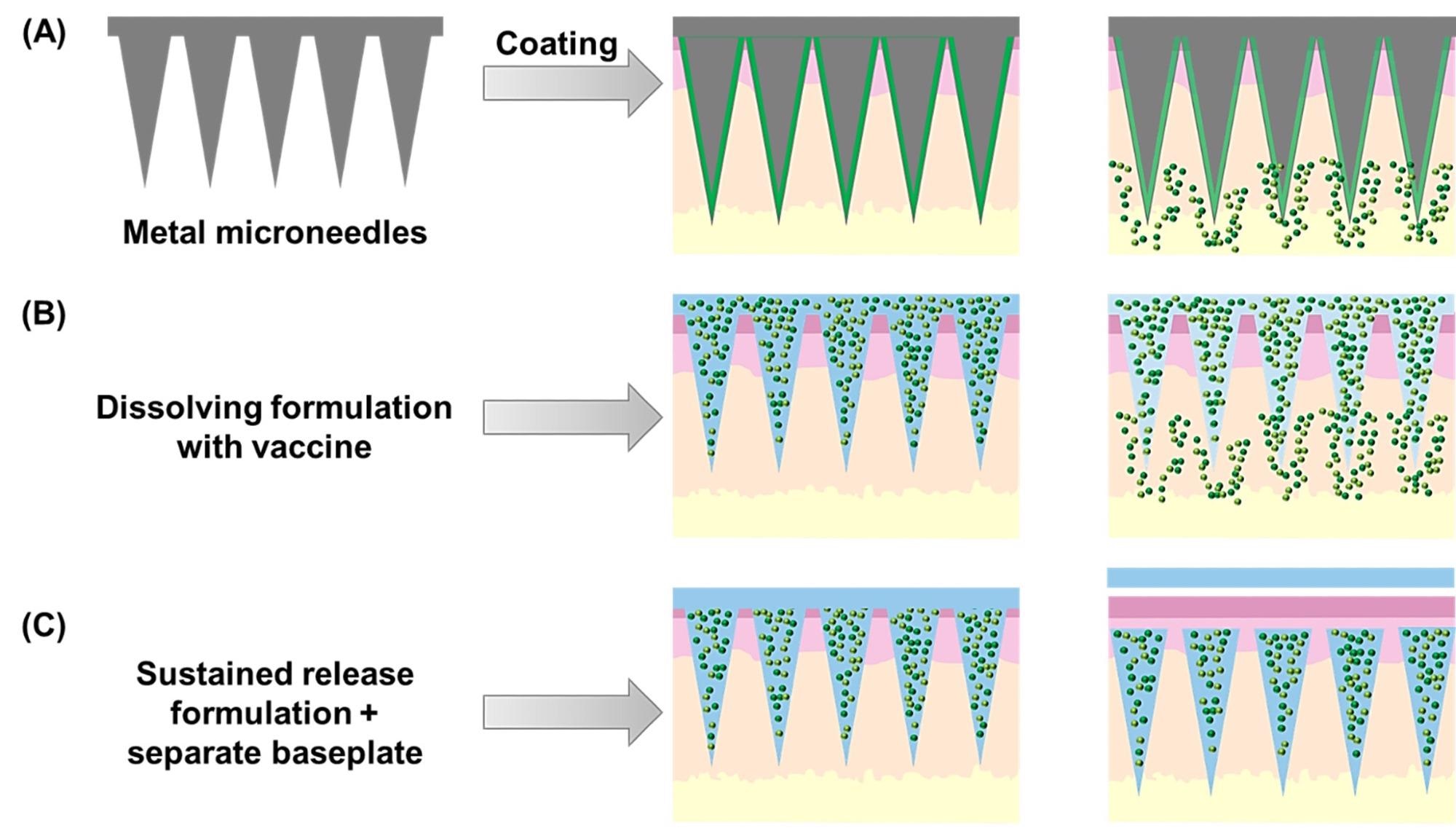
Schematic representation of the three main types of microneedle arrays developed for vaccine delivery. Coated microneedle arrays (A) are prepared using a solid array base, usually metallic, which is coated with a dissolving formulation containing the antigen(s) and adjuvant(s). Alternatively, dissolving formulations (B) can be used to manufacture the entire array, leading to vaccine delivery upon skin insertion of these self-disposable devices. Finally, sustained-release formulations (C) have been used to produce implantable microneedle tips that are left in the skin after insertion, upon removal of a separate baseplate. Image Credit: Patil-Sen, Y. et al.
Microneedles for vaccine administration have also been extensively explored, both preclinically and clinically.
With these samples, promising findings have been produced, with microneedles achieving equal effectiveness, safety, and stability to IM vaccine delivery. These methods have enormous promise for facilitating mass vaccination campaigns, especially in low-income areas, and might have a substantial influence on vaccine delivery and coverage in the coming years.
Future Perspective
Although nanoparticles-based drug delivery systems improve vaccination effectiveness, the safety and stability characteristics of both nanocarriers and antigenic components should be closely reviewed in the context of the regulators' requirements.
A general regulatory consensus on nanovaccine reliability, safety, and effectiveness would allow a seamless approval procedure for safe and effective treatments.
Continue reading: Post-Pandemic Outlook for Antiviral Nanoparticles and Nanofiber Filters.
References
Patil-Sen, Y. et al. (2021) Nanovaccine Delivery Approaches and Advanced Delivery Systems for the Prevention of Viral Infections. Pharmaceutics, 13(12), 2091. Available at: https://www.mdpi.com/1999-4923/13/12/2091
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.