The consistent heterostructure interfaces were achieved by optimizing the nitriding extent, followed by an efficient modification of the energy band structure. The sulfur specimen reaction kinematics of the graphene composite sulfuric cathode supplemented by these nanowires are enhanced.
Drawbacks of Lithium-Sulfur Cells
Owing to their significantly greater theoretical capacity and energy density as compared to typical Li-ion batteries, lithium–sulfur (Li–S) batteries have received a lot of interest.
Transitional polysulfides (Li2Sn, n = 4–8), on the other hand, may disperse in the electrolytic solution and accumulate on the opposing electrode, producing the "shuttle effect." This would deplete electroactive substances, which will reduce capacity and Coulombic efficiency.
High volumes of polysulfides interact with lithium anode dismutation, weakening the electrode interface's integrity and considerably increasing the risk of Li dendrites. Furthermore, the unimpressive electron conductance of sulfur and the sluggish reaction kinetics significantly impede the quick start-up and responsiveness of Li–S cells.
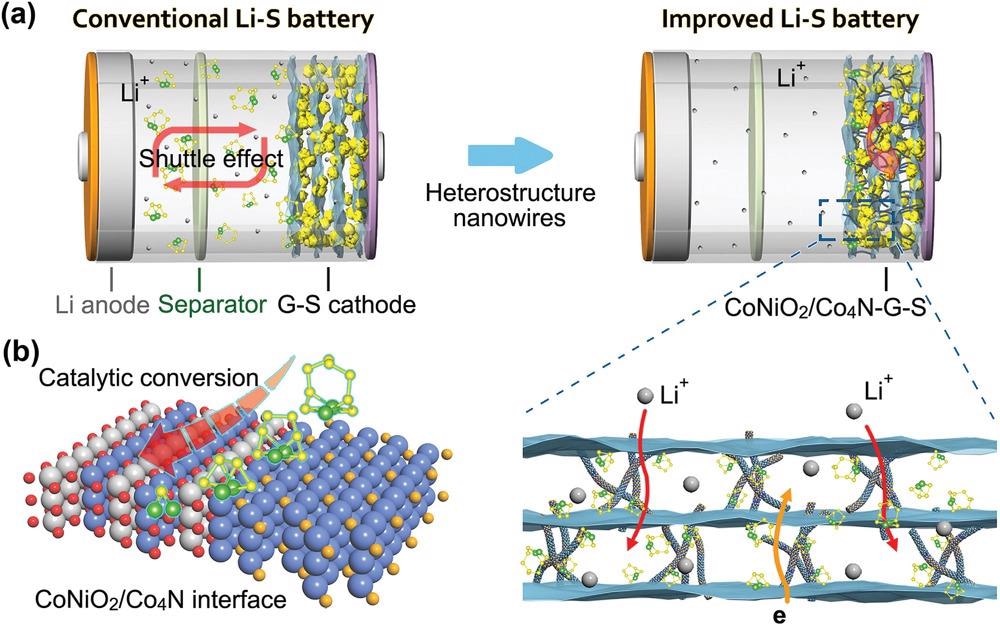
a) A comparison of the conventional Li–S battery with “shuttle effect” and the CoNiO2/Co4N based improved cell. b) Schematic illustration of interface mechanism of heterostructure and polysulfides. © Pu. J., et al (2021)
Electrodes based on Graphene–Sulfur Composites
Several studies have investigated novel electrode materials and structures to address these concerns. Among the key methods is to disperse active sulfur into a high porosity matrix of carbon to generate a composite cathode.
Graphene, a carbon-based substance, is often used due to its high conductance and large specific area. Because of this, it is employed as an electrical network to lower sulfur interface impedance and as a physical obstacle to prevent diffusion of polysulfides, respectively. However, numerous fundamental flaws in graphene–sulfur (G–S) composite structures remain.
The nonpolar carbon surface has a weak attraction towards polar hydrophilic polysulfides. As a consequence of this poor capturing ability, cyclic stability is restricted, and sulfur loading is considerable.
Altered graphene with functional groups (such as hydroxyl and carboxyl) may improve polysulfide adsorption. However, it will diminish the material's conductance and increase the manufacturing intricacy. Furthermore, graphene's agglomeration and layering features must be noted since they may provide unnecessary impediments to ionic transport.
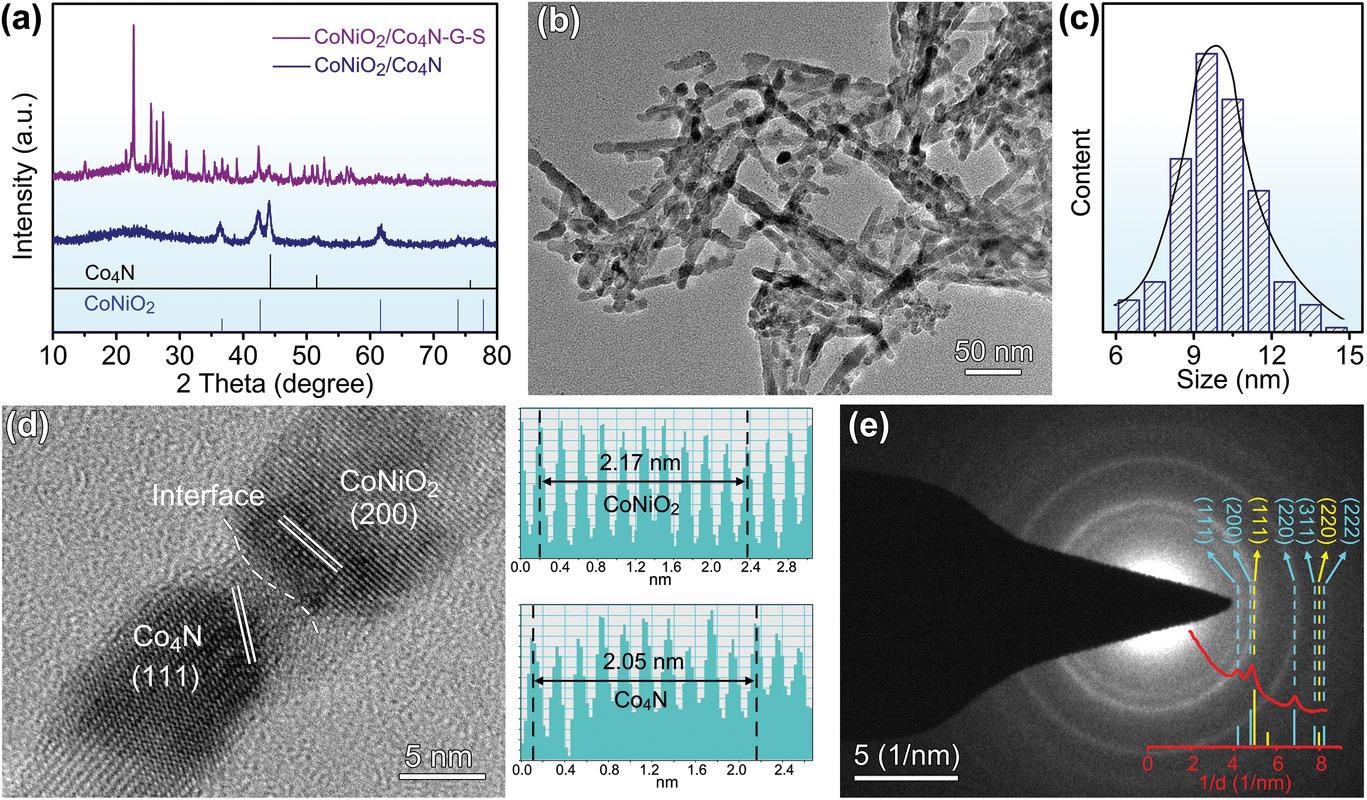
Phase characterization of CoNiO2/Co4N heterostructure nanowires. a) XRD patterns. b,c) TEM image and diameter distribution. d) HRTEM result and corresponding lattice spacing. e) SAED pattern and rotational integral.© Pu. J., et al (2021)
Incorporating Transition Metal Compounds with Graphene
The coupling of polar transition metal compounds (TMCs) such as nitrides and oxides with graphene has shown to be an efficient technique for inhibiting the shuttle effect and increasing Li2Sn transformation. However, TMCs typically only have single or dual functions that are relatively simple and incapable of incorporating all benefits.
To address this issue, researchers have recently focused on constructing TMCs with distinct functionalities into a cohesive heterojunction framework. Though the heterostructures created to date have outstanding cathode performance due to synergistic effects, the sizable structure (size >100 nm) restricts the total energy density increase. This occurs because these inert supplements do not give extra reaction capacity; only surface films perform adsorptive and catalytic processes. Excess use might raise the battery's total weight. Thus, designing heterojunction architecture is critical.
In addition to conductive, catalytic, and adsorptive characteristics, another property of an optimum G–S cathode supplement is the minimal use of materials. Although extremely fine nanoparticles and extremely thin nanosheets are strong structural solutions, none can effectively address the problem of graphene aggregation.
Previous research has demonstrated that introducing a 1D framework may successfully reduce graphene aggregation. Also, the high aspect ratio design has a reduced entry barrier and is conducive to developing a consistent conductive matrix. So, building heterojunctions using TMC nanowires as a graphene-based sulfur cathode medium shows great promise.
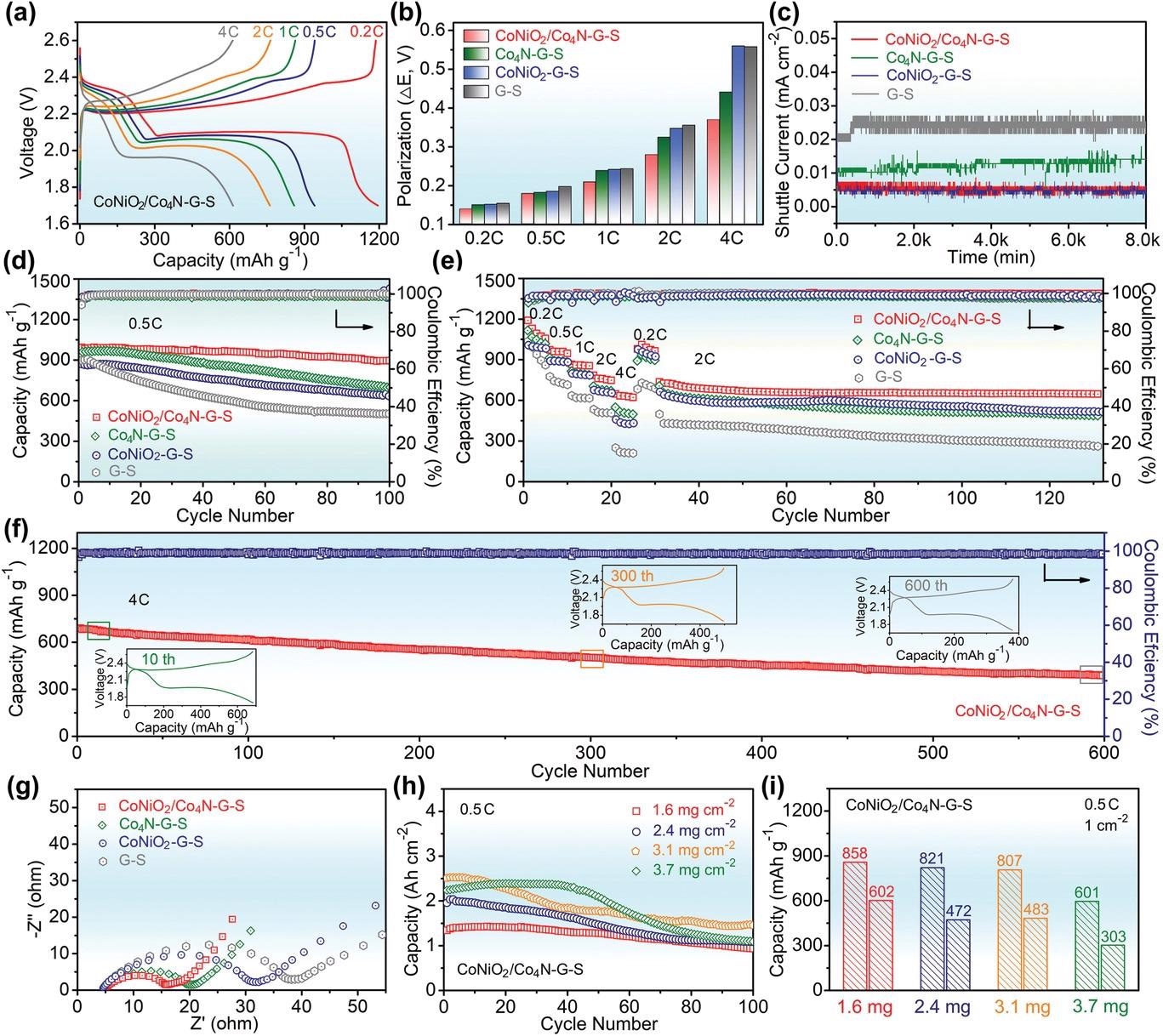
Electrochemical performance of CoNiO2/Co4N–G–S and other control cathodes. a) Charge–discharge profiles of CoNiO2/Co4N–G–S at various current densities. b) Comparing the potential polarizations of different electrodes. c) Shuttle current test. d) Cyclic properties of different cathodes at 0.5 C. e) The rate conversion and the subsequent constant current cycle. f) Cyclic stability of the CoNiO2/Co4N–G–S electrode at high rate. g) EIS curves at 20th cycle. (h,i) Cyclic performance of CoNiO2/Co4N–G–S with different sulfur loading at 0.5 C. © Pu. J., et al (2021)
Summary of the Research Findings
The team generated a CoNiO2/Co4N heterojunction nanowire matrix to reduce polysulfide incompatibilities and kinetic latency of graphene host of Li–S cells. It paired CoNiO2's powerful polysulfide attachment capability with Co4N's good electric conductance, while the heterogenized interface showed a greater aptitude as the active site for ionic transport and polysulfide conversion.
The nanowire framework in this approach would successfully prevent graphene mounting and aggregation. By incorporating Co4N, the energy band structure of CoNiO2 was finely adjusted, significantly improving electric conductance. Making use of the interface effect, the resulting heterojunction kept the increased polysulfide adsorption and electrocatalysis of CoNiO2, minimizing the "shuttle effect".
Meanwhile, the Li-ion diffusion behavior of the CoNiO2/Co4N–G–S cathode was more pronounced. As a result of the reduced polysulfide diffusion and better electrochemical reaction kinetics, the overall quality of the battery was improved. Thus, the Li–S battery based on CoNiO2/Co4N exhibited high-rate performance, good reversibility capacity, and outstanding cycle stability along with reduced capacity degradation.
Continue reading:Solid Mercury Nanoparticle Synthesis Suggests Sustainable Future for Electroanalytical Chemistry.
Reference
Pu, J., Gong, W. et al. (2021) CoNiO2/Co4N Heterostructure Nanowires Assisted Polysulfide Reaction Kinetics for Improved Lithium–Sulfur Batteries. Advanced Science. Available at: https://onlinelibrary.wiley.com/doi/10.1002/advs.202104375
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.