Here, new insights have emerged into nanoparticle vaccines' design and synthesis to resist recurring and pandemic influenza (flu) and other dangers of respiratory infectious disease.
Influenza, the Most Serious Infectious Respiratory Diseases
One of the most serious and infectious respiratory contagious diseases is influenza. Infection with the influenza virus creates annual outbreaks and occasional pandemics, leading to a huge health and economic burden worldwide.
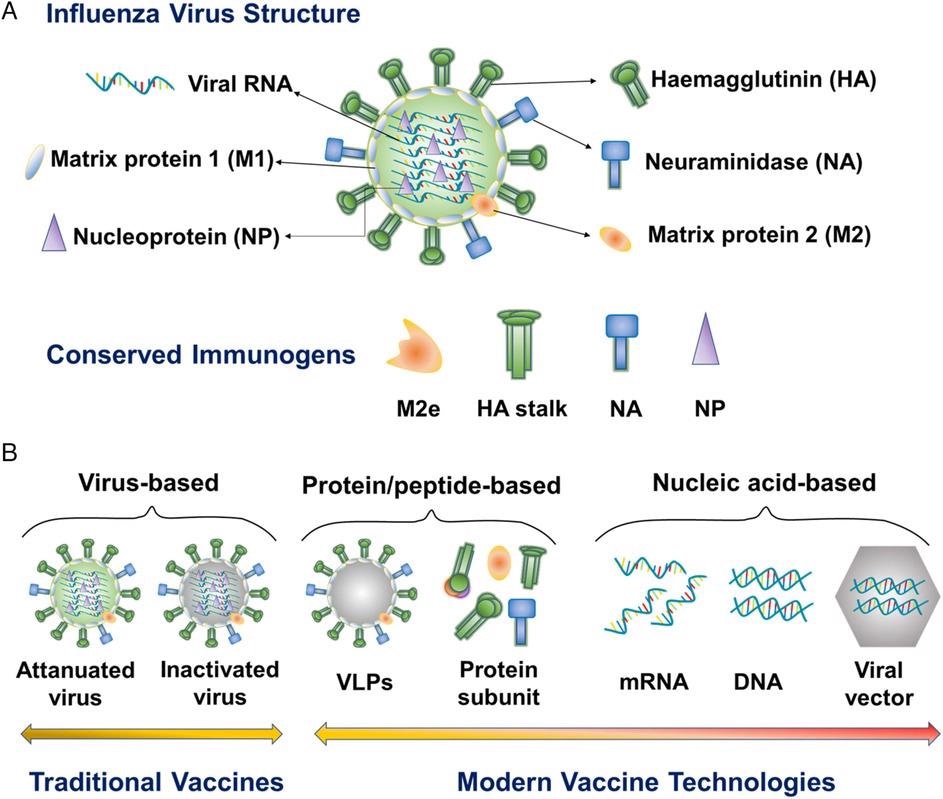
Schematic illustration of influenza virus structure, conserved influenza immunogens, and modern influenza vaccine types. M2e, M2 extracellular domain. VLP, virus-like particle. © Dong, C., and Wang, B. (2021)
Flu viruses are RNA viruses with an envelope that belong to the Orthomyxoviridae family.
Present seasonal flu vaccines induce strain-specific immunity that quickly wears off, have low efficiencies against misaligned circulating strains, and has no effect on epidemics.
Next Generation of Vaccine with Nanotechnology
Restrictions of existing influenza vaccines hope to be overcome by a next-generation widespread influenza vaccine that evokes long-lasting cross-protective protection against variant flu strains.
Nanotechnology is essential for the implementation of such vaccines.
Nanoparticle vaccines can best mimic the seasonal influenza nanostructure with elevated antigen loads, allowing for targeted delivery and controlled antigen release and collaborating with single-molecule adjuvants to increase vaccine effectiveness and breadth.
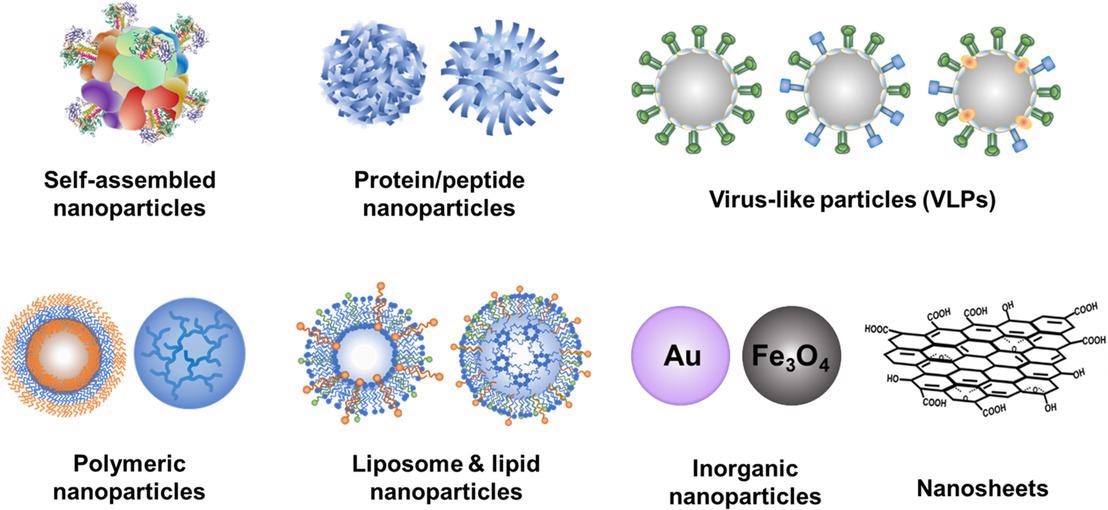
Various nanoparticle platforms for vaccine development. © Dong, C., and Wang, B. (2021)
Furthermore, nanoparticle vaccines can enable rapid and scalable manufacturing, reduce cold-chain dependence, implement antigens flexibly modularly, and be generated at a low cost. To date, several nanoparticle formulas have demonstrated promising results in terms of creating cross-reactive seasonal flu immunity.
Development of Influenza Vaccines
To reduce the public health consequences of seasonal and pandemic influenza, the development of a vaccine that provides long-term cross-protection against numerous flu viruses becomes a top priority. The recognition of viral proteins by the host immune system is required for the induction of a protective immune response against influenza.
Seasonal flu vaccine induces strain-specific antibody production to the HA head's high variability immune determinants. These kinds of antibodies are frequently neutralizing and efficient in combating viral replication, but they are susceptible to immune evasion.
Significant advancements in flu immunology and vaccinology suggest that universal flu vaccines targeting correlated immune determinant factors and providing broad protection against variant strains are viable, though challenges remain. The development of universal influenza vaccines requires the design and characterization of novel immune aims as immunogens.
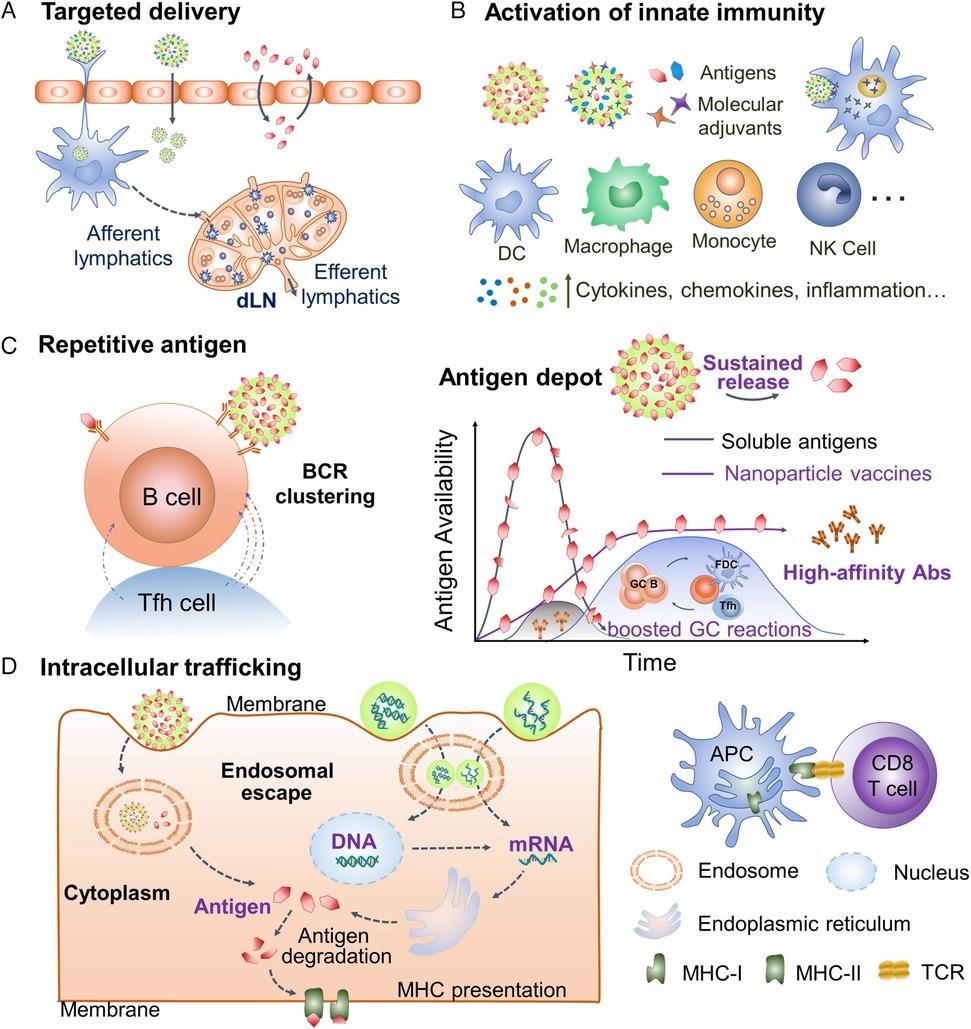
Intriguing characteristics of engineered nanoparticle vaccine platforms. a) Nanoparticles facilitate targeted antigen/adjuvant delivery to dLNs and immune cells. b) Nanoparticles cooperate with molecular adjuvants to induce innate immunity. c) The nanosize, high antigen loads, and controlled antigen releases enhance B cell activation and GC reactions, producing high-affinity antibody responses. d) Nanoparticles trafficking cargos into the cytoplasm facilitate cross-presentation of exogenous antigens and delivery of nucleic acid vaccines. dLNs, draining lymph nodes. GC, germinal center. Abs, antibodies. bnAbs, broadly neutralizing antibodies. NK, natural killer. MHC, major histocompatibility complex. TCR, T-cell receptor. © Dong, C., and Wang, B. (2021)
Challenges of Developing Vaccines
However, significant difficulties have been encountered in developing a HA-stalk-based universal influenza vaccine: 1) the complexity in designing and building and expressing the HA stalk antigens besides other HA sequences; 2) the reduction immunogenicity of subunit vaccines when compared to particulate antigens; and 3) the comparatively short duration of the immunity induced.
Nonetheless, several studies have shown that HA stalk region sequences, especially those found on nanoparticle vaccines, can be used as immunogens in the development of broadly protective influenza vaccines.
Nucleic Acid Vaccine as the Most Recent Advancement
The latest advancements in vaccine technology are nucleic acid (mRNA and DNA) vaccines. Nucleic acid vaccines are noninfectious and can enhance potent immune responses by using host cellular machines and equipment to produce encoded protein antigens.
Specifically, mRNA vaccines are less dangerous than DNA vaccines since they can be deteriorated naturally and do not integrate into the host's genomic DNA. RNA methods of production, such as the use of modified nucleosides, have enhanced mRNA resistance to RNases.
Self-amplifying replicon RNA could be used to achieve long-term antigen expression. The extraordinary success of the SARS-CoV-2 (COVID-19) mRNA vaccines promotes the development of mRNA vaccines against other infectious diseases, such as influenza.
Favorable New Technology, Nanoparticles and their Advantages
One of its most favorable technological innovations for developing next-generation influenza vaccines is the nanoparticle vaccine framework. Nanoparticles can house numerous vaccine components (proteins, peptides, DNA, mRNA, and molecular adjuvants) in a reconfigurable, tunable, and flexible manner, facilitating the development of low-cost vaccines by promoting organizationally simplified vaccine manufacturing, collection, distribution, and administration.
Tailor-made nanoparticle vaccines can program the initiation of potent broadly protective immune function while also allowing for antigen dose reduction. Tailored nanoparticle vaccines can process the induction of potent broadly protective immune function while also allowing for antigen dose reduction and become effective for influenza.
Continue reading: Improving Infectious Diseases Treatment with Nanotechnology: A Review.
Reference
Dong, C., and Wang, B. (2021) Engineered Nanoparticulate Vaccines to Combat Recurring and Pandemic Influenza Threats. Available at: https://doi.org/10.1002/anbr.202100122
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.