One of the main causes of water pollution is the contamination of water bodies by industrial effluents. The different types of water contaminants include organic and inorganic dyes that are used as colorants in many industries, such as food, cosmetics, paper, and textiles.
Nanomaterials are tiny particles whose size ranges from 1-100 nm and possess unique physical, chemical, electrical, optical, catalytic, and biological properties.
These particles have a high surface to area ratio and are applied in varied fields of science and technology. Silver (Ag) nanoparticles (NPs) are among the most widely used nanoparticles owing to their superior catalytic activity and antimicrobial properties.
Green Synthesis of Nanoparticles
Various methods are used to synthesize Ag NPs, such as chemical, radiation, electrochemical, and biological methods.
Green synthesis of Ag NPs is a biochemical-based process that utilizes living organisms (e.g., plant, bacteria, algae, or fungi) or their metabolites, as the reducing agent.
Typically, nanoparticles are produced by first reducing the salt containing the metal ion. Following this, the newly synthesized nanoparticles are stabilized via capping techniques.
Phytochemicals extracted from plants and secondary microbial metabolites are often used as reducing and capping agents.
The main advantage of the green synthesis of nanoparticles is that it does not use any hazardous chemicals and does not produce any harmful by-products.
Additionally, this method is cost-effective, eco-friendly, and does not require any stabilizers. Scientists have determined the metabolites, such as alkaloids, that are responsible for the conversion of metallic Ag to Ag nanoparticles.
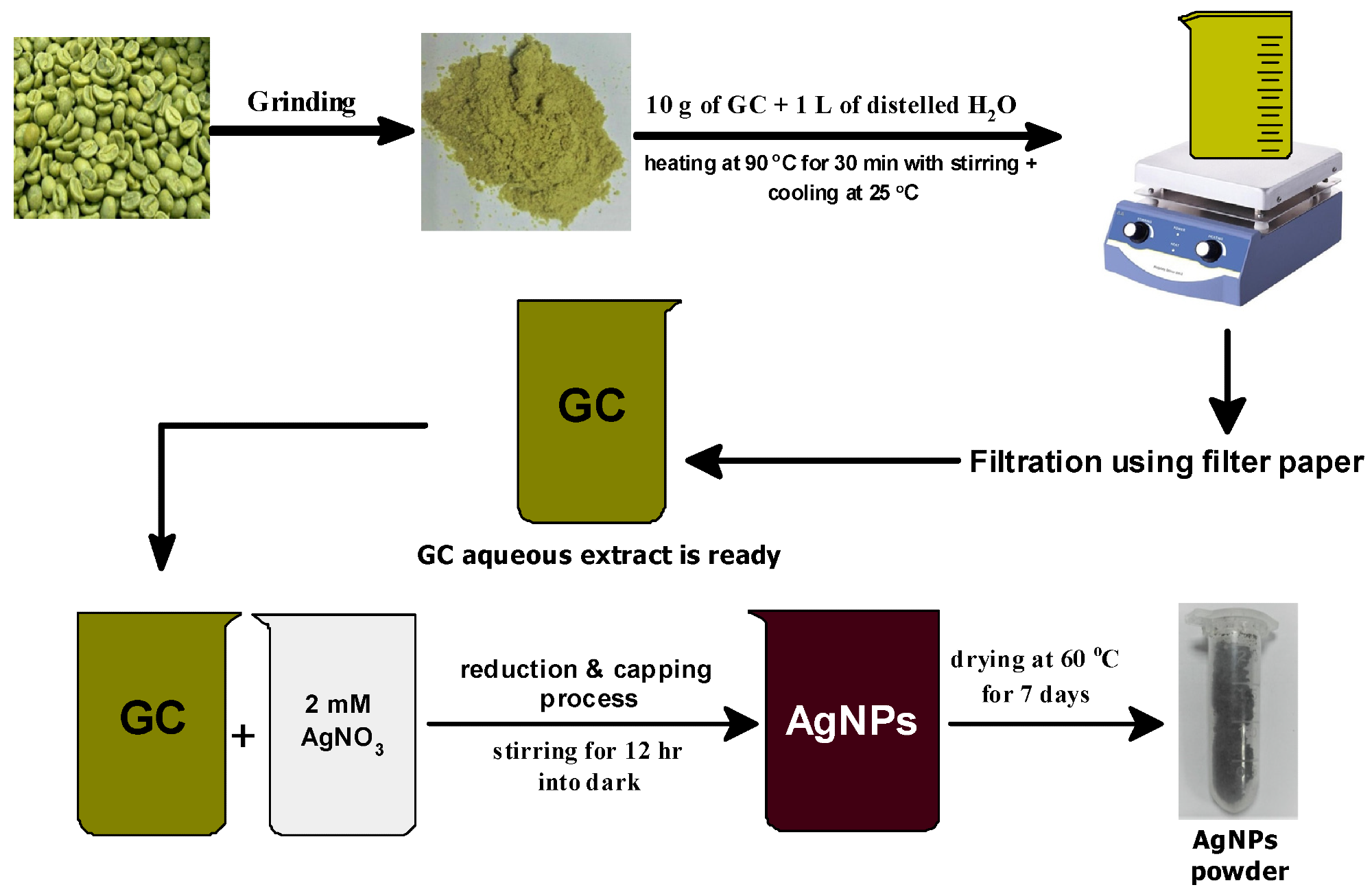
Figure 1. Schematic diagram of the preparation method of phyto-capped Ag NPs. © Kordy, M.G.M. et al. (2022)
Green Synthesis of Silver Nanoparticles - A New Study
Coffea arabica is one of the most popular plants, whose beans are used regularly to produce coffee.
In the new study, aqueous extracts of Arabic green coffee (GC) beans were used as the reducing and stabilizing agent for the synthesis of silver nanoparticles.
Researchers investigated the ability of these Ag NPs as antioxidants and catalysts for methylene blue dye reduction by sodium borohydride.
Previous studies have revealed that GC extracts contain many important phytoconstituents such as alkaloids, glycosides, and other phenolic compounds, that can convert metallic silver to silver nanoparticles.
The mechanisms behind the production of silver nanoparticles using GC extract are discussed in the following steps.
- The Ag atoms are formed by the reduction of Ag+ ion by GC extract, which nucleates to form Ag NPs.
- The size of the nanoparticles is controlled using electrostatic stabilizing agents via the capping process. This process requires a stabilizer that can adsorb onto the surface of the newly synthesized Ag NPs to be capped. The authors used the GC extract as a capping agent as well.
The results of this study are in line with previous studies where researchers have used various plant extracts such as Emblica officinalis (amla), Aloe vera, and Phyllanthus emblica (Indian gooseberry).
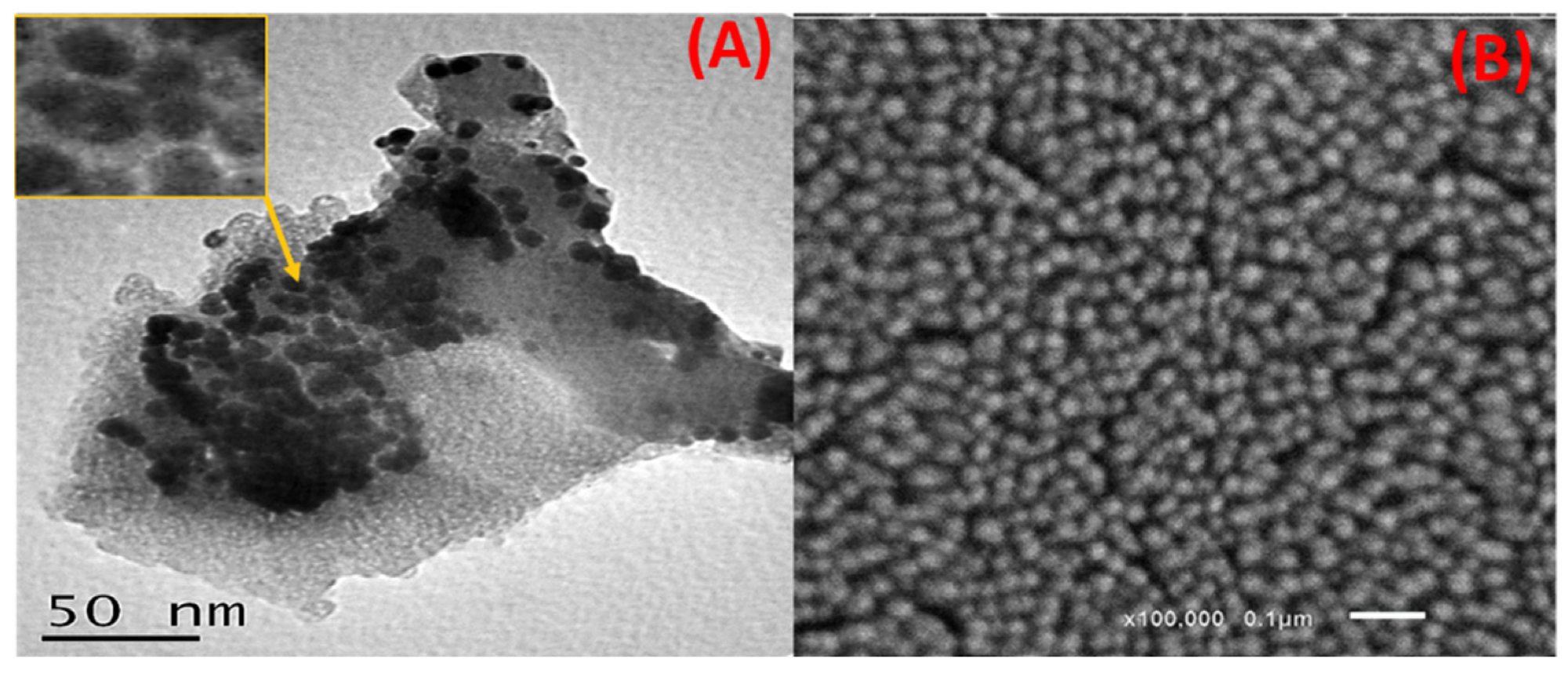
Figure 2. (A) HRTEM and (B) SEM images of the capped Ag NPs. © Kordy, M.G.M. et al. (2022)
Characterization of Newly Synthesized Ag NPs
Scientists used various analytical tools such as SEM, EDX, FTIR, TEM, DLS, zeta potential, and XRD to characterize the biologically synthesized Ag NPs using GC extract.
Initially, the production of Ag NPs was determined by the color change of the reactants from pale yellow to dark brown.
The synthesis of Ag NPs was further determined by a UV-Visible spectrophotometer where the researchers detected the surface plasmon resonance (SPR) of GC-capped Ag NPs at 425 nm.
The difference in the FTIR spectrum of Ag NPs as well as GC extract signifies the participation of polyphenolic compounds in the reduction process.
FTIR analysis indicated the role of natural metabolites in the reduction of Ag+ and the stabilization of the green-produced Ag NPs. TEM and SEM analysis revealed that Ag NPs were poly-dispersed and were spherical or semi-spherical in shape.
The EDX spectrum also confirmed the presence of Ag signals at 2.983 keV. The crystalline nature of GC-capped Ag NPs was determined using XRD analysis.
The zeta potential of the colloidal sample determined the stability of the Ag NPs.
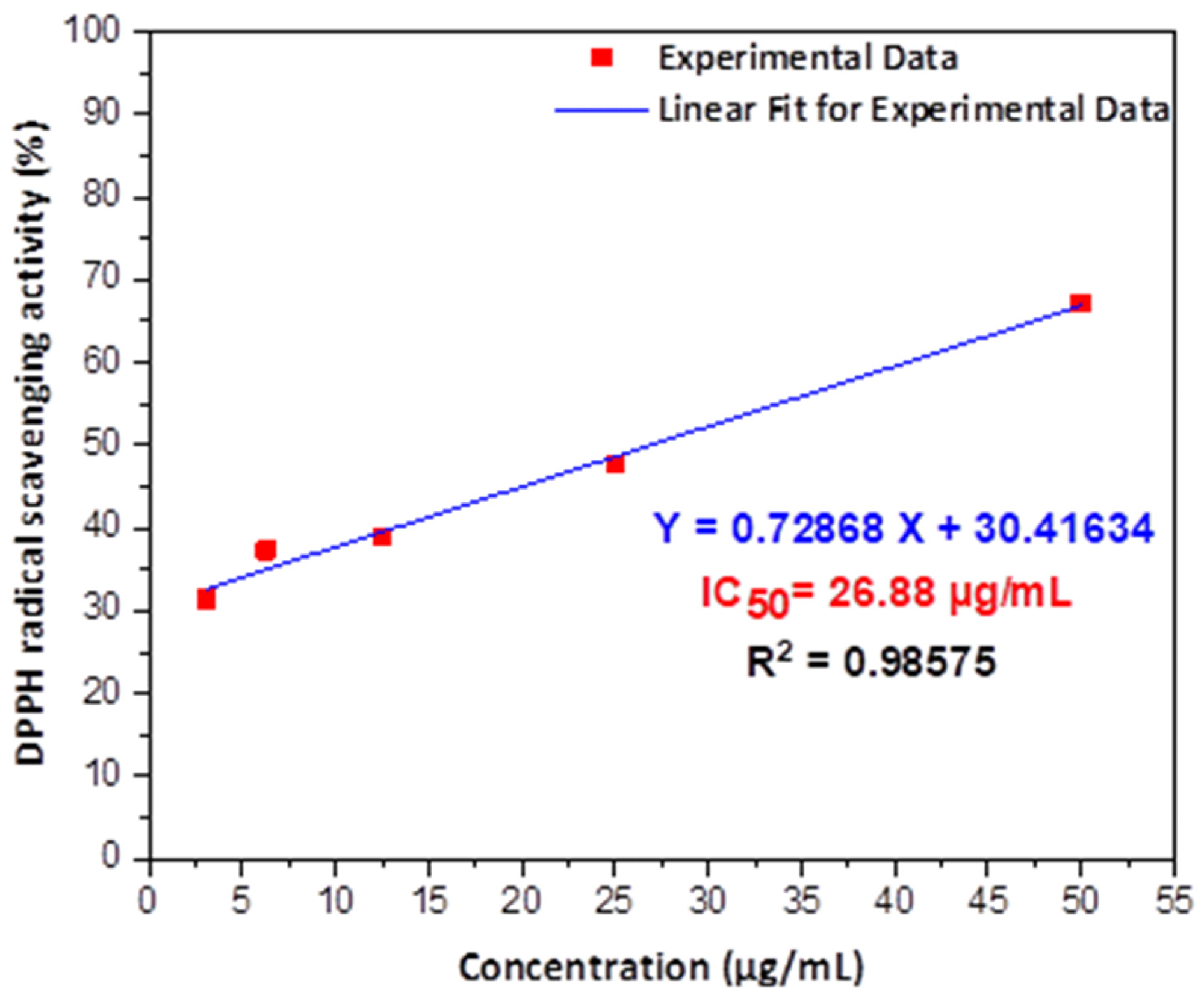
Figure 3. The antioxidant activity of GC-capped Ag NPs using different concentrations to determine the IC50 after linear fitting for experimental data. © Kordy, M.G.M. et al. (2022)
Reduction of Methylene Blue Dye Using Ag NPs
In this study, scientists determined the potential of the newly developed Ag NPs as a reducing agent of methylene blue dye in the presence of sodium borohydride (NaBH4).
They reported a high degradation efficiency of 96% by GC-capped Ag NPs. Researchers revealed a catalytic reduction of 50 ppm of MB dye using 0.1 M of NaBH4.
Additionally, maximum catalytic activity was achieved in a record time of 12 minutes.
A robust antioxidant activity has been reported for the first time against 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals.
Scientists are optimistic that GC-capped Ag NPs could be effectively applied in the treatment of contaminated water in the future.
Continue reading: How are Nanocatalysts Used for Environmental Applications?
Reference
Kordy, M.G.M. et al. (2022) Phyto-Capped Ag Nanoparticles: Green Synthesis, Characterization, and Catalytic and Antioxidant Activities. Nanomaterials. 12(3):373. https://doi.org/10.3390/nano12030373
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.