In this context, a low-cost processing approach is required to keep the prices as low as possible since lithium is substituted with sodium mainly for economic reasons.
A recent study published in the journal Scientific Reports addresses this issue by creating an ultra-fast synthesis technique of sodium iron phosphates-carbon (NaFePO4-C) nanocomposites. This strategy utilizes the high microwave absorption of silicon carbide concentration in rice straw ash.
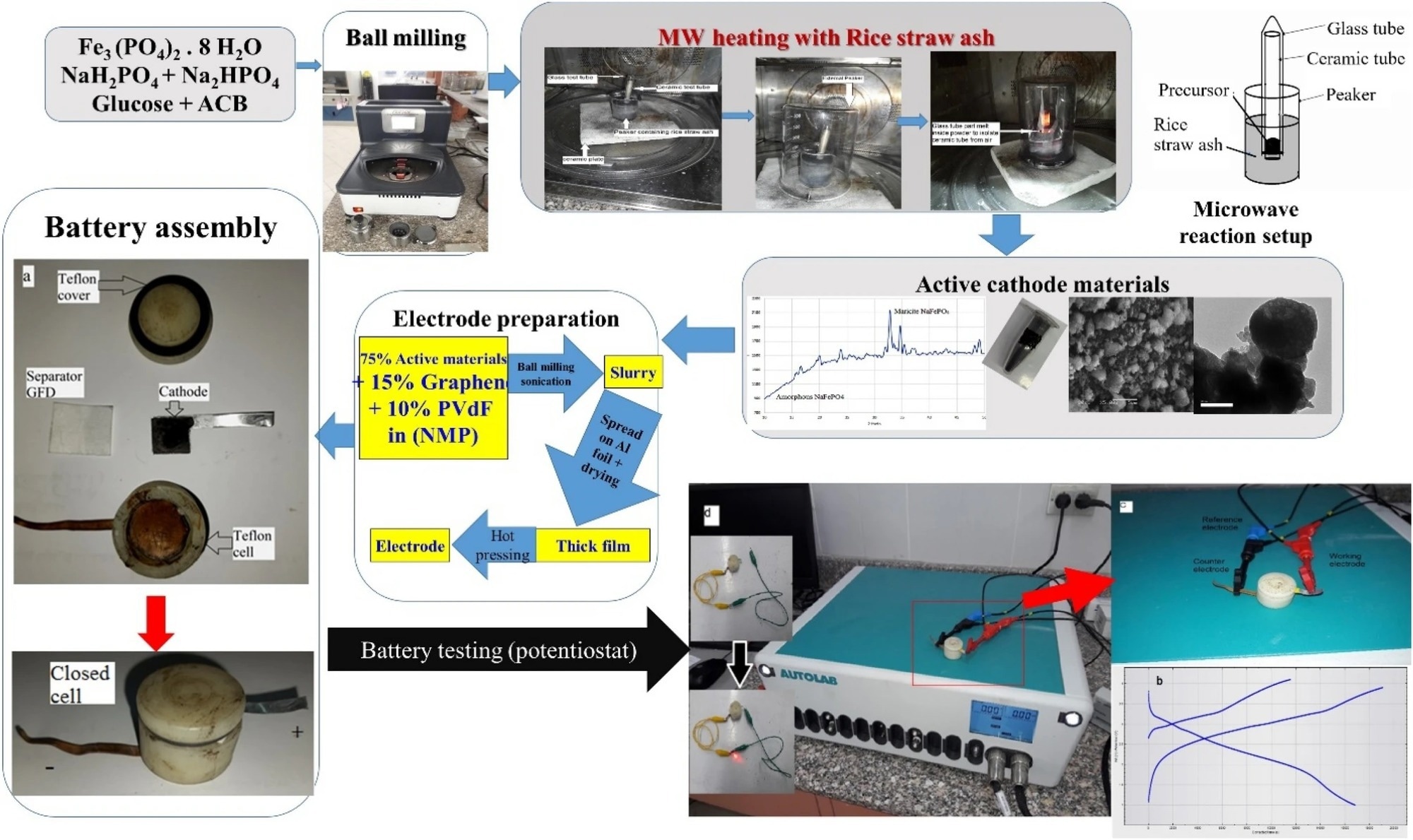
Figure 1. Synthesis process of sodium iron phosphate through ball milling and microwave assisted and assembly of related batteries and supercapacitors. © Wazeer, W. et al. (2022).
Sodium-ion Batteries and Supercapacitors: Overview and Significance
Lithium-ion devices have infiltrated many energy storage applications, such as electric cars, laptops, and mobile phones because of the relatively high gravimetric capabilities of lithium batteries. However, lithium is scarce and prohibitively costly, forcing the hunt for suitable substitutes.
Sodium-ion batteries and supercapacitors are important rechargeable storage technologies that work similarly to lithium-ion batteries but employ sodium ions (Na+) as charge carriers. The operating principles and cell structure of these batteries are almost comparable to those of commercially available lithium-ion batteries.
Many properties of sodium are remarkably similar to those of lithium, including its electron affinity and atomic radius. Sodium is a thousand times more plentiful than lithium and has an almost unlimited supply, with significantly cheaper total extraction and purifying costs.
Sodium-ion batteries and supercapacitors are more environmentally friendly, safer, and have lower raw material prices than lithium-ion batteries. Moreover, sodium-ion batteries and supercapacitors promise high performance, theoretical capacity, and cycle rate.
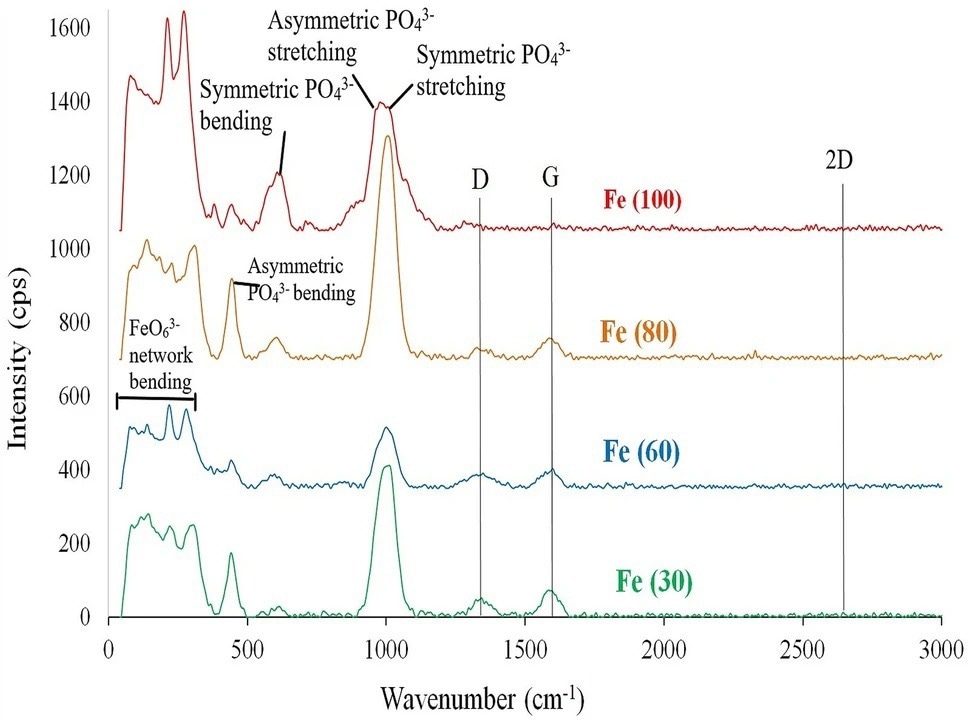
Figure 2. Raman spectra for microwave solid state synthesized sodium iron phosphate samples. © Wazeer, W. et al. (2022).
Nanocomposites for Sodium-Ion Batteries and Supercapacitors
Several nanocomposites have been identified as electrode materials for sodium-ion batteries and supercapacitors.
Sodium vanadium phosphates (NVPs) are some of the most effective electrode materials for sodium-ion batteries and supercapacitors because of their high discharge potential energy density. They also offer a high-rate capacity due to their sodium ion superconductive framework.
However, only two of the three sodium ions of NVPs are accessible at this potential, resulting in a limited theoretical capability. Moreover, these oxides can be expensive compared to other sodium nanocomposites.
Iron oxide is available at one-tenth the price of vanadium oxide as a raw material; therefore, sodium iron phosphate can be used as a low-cost cathode material for sodium-ion batteries and supercapacitors. Maricite and olivine are crystalline forms of sodium iron phosphate that have been investigated as effective and cheap cathode materials.
Highlights of the Current Study
Microwave solid-state technologies allow for the rapid and low-cost production of alkali metallic phosphate nanocomposites. Although these procedures have been routinely employed to create olivine lithium-iron phosphate nanocomposites, they are seldom used to produce sodium-transition metal phosphates.
In this study, the researchers described an ultra-fast synthesis technique that uses the high microwave absorption of silicon carbide in rice straw ash. Using a one-step microwave heating process, amorphous solutions of sodium iron phosphates-carbon nanocomposites (NaFePO4-C) were produced, annealed, and carbon-coated.
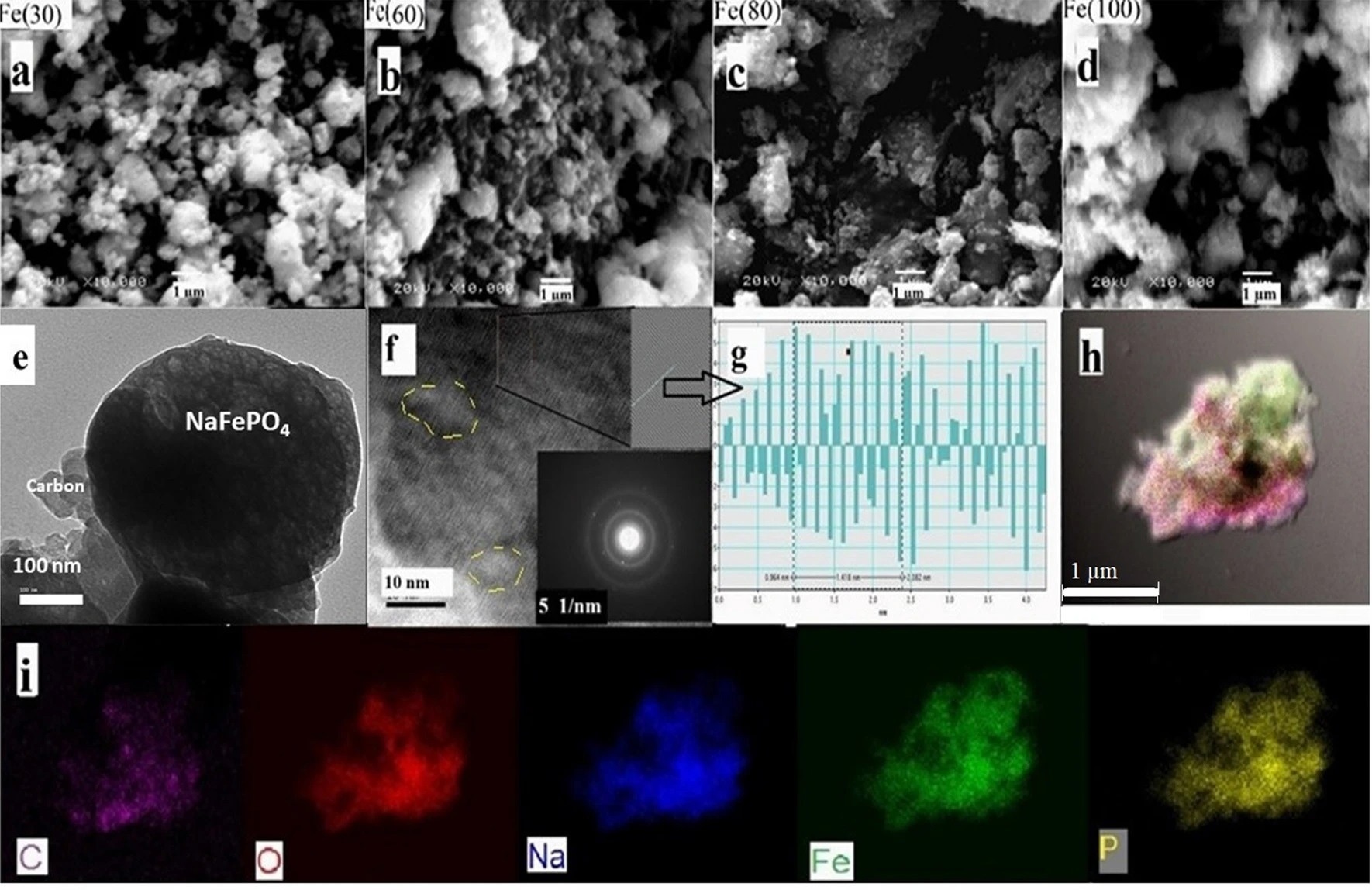
Figure 3. (a–d) SEM images at ×10,000 for Fe(30), Fe(60), Fe(80) and Fe(100) respectively. (e) Low magnification TEM image showing carbon compositing with NaFePO4 particles, (f) High magnification TEM image and SAED image for NaFePO4 spot, (g) FFT images and IFFT profile of Fe(60) for selected area along (240) plane and (h,i) Elemental mapping showing C, O, Na, Fe, and P distribution on the selected particle. © Wazeer, W. et al. (2022).
The sodium ion electrocatalyst composites were made with microwave heating times ranging from 30 to 100 seconds.
Elemental analytical methods, X-Ray diffraction (XRD), scanning and transmission electron microscopy (SEM, TEM), and Raman spectroscopy were used to analyze the nanocomposites. The electrocatalytic activity of the produced nanocomposites as cathodes for sodium-ion batteries and symmetric supercapacitors was also investigated.
Key Developments of the Research
Egyptian rice straw ash was used as a microwave absorber to facilitate production in a relatively short period and at a reasonable cost. This quick microwave-assisted heating with slightly reducing conditions eliminated the requirement of argon gas in the synthesis, precipitation, and carbon coating stages.
Moreover, this process is adaptable, allowing for the use of a wide range of sodium, ferrous, phosphate, and carbon sources and optimizing the storage capacity, stability, and cyclic performance of sodium-ion batteries.
The disadvantage of this process is that the nanocomposites produced are uneven and non-uniform in shape and size. The microwave produces polymorphic substances with tiny crystallite sizes. The crystal formation cannot be completed because of the rapid heating, and only 10-15 nm particle-size nanocomposites can be formed, as revealed by TEM.
However, the nanocomposites aggregate, generating bigger grains with an average size of around 300 nanometers, limiting the sodium ion diffusivity. As a result, the produced nanocomposites demonstrated excellent electrocatalytic behavior as cathodes for sodium-ion batteries and supercapacitors.
Reference
Wazeer, W. et al. (2022). Ultra-fast green microwave-assisted synthesis of NaFePO4-C nanocomposites for sodium-ion batteries and supercapacitors. Scientific Reports. Available at: https://doi.org/10.1038/s41598-022-20329-x
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.