In addition to the antiviral potential of imprinted nanoparticles against the wild-type SARS-CoV-2 variant, they also exhibited broad-spectrum antiviral efficacy against other variants, including N501Y, D614G, Δ69-70, N439K, omicron, and delta. Thus, the present work has paved a new path toward the viral glycan shield targets against the wild-type SARS-CoV-2 and delta variant, exhibiting a broad spectrum of viral inhibition.
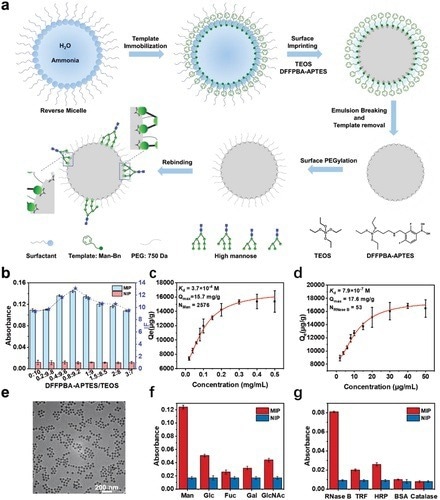
Figure 1. Design and characterization of nanoMIP. a) Preparation procedure of nanoMIP. b) Optimization of imprinting conditions. c,d) Binding isotherms and affinity measurement of nanoMIP at the monosaccharide and protein level, respectively. e) Representative TEM image of nanoMIP. f,g) Selectivity test, comparison of the UV absorbance for test monosaccharides (at 230 nm) and proteins (at 214 nm) captured by nanoMIP and corresponding NIP. Mean ± SD, n = 3.
SARS-CoV-2 Variant and Molecularly Imprinted Nanoparticles
The onset of COVID-19 has severely affected global health and socioeconomic development. Although various prophylactic and therapeutic strategies have been developed, the constant mutation of viruses poses significant challenges.
In particular, the constantly emerging SARS-CoV-2 variant is becoming resistant to the current vaccines and therapeutics. Moreover, the spike of recent variants of concern omicron (B1.1.529) undergoes approximately 30 mutations, becoming remarkably resistant to several antibodies and causing a recurrence of infection. Thus, broad-spectrum inhibitors against mutated SARS-CoV-2 variants are highly desirable.
Molecularly imprinted polymers (MIPs) are tailored polymeric receptors prepared by chemical synthesis that contain receptors with antibody-mimicking binding properties. MIPs offer several advantages, including cost efficiency, ease of preparation, and storage stability.
Thus, nanoparticles synthesized from MIPs are referred to as plastic or artificial antibodies. Although MIPs are efficient in exhibiting great potential in disease diagnosis, toxin neutralization, virus recognition, and cancer therapy, there have been no reports of MIPs showing their broad-spectrum virus inhibitory efficacy.
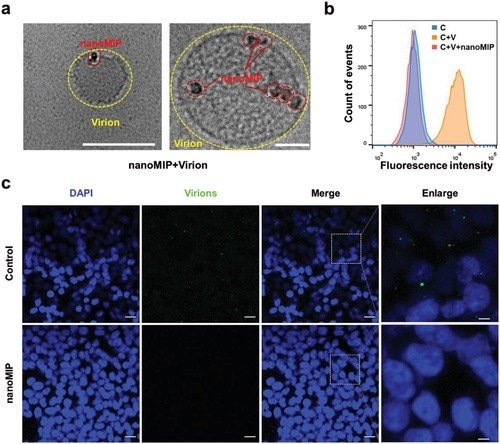
Figure 2. Inhibition of SARS-CoV-2 pseudovirus (lentiviral pseudoparticles) attachment to ACE2-HEK293T cells by nanoMIP. a) Cryo-transmission electron microscopy images for the SARS-CoV-2 pseudovirus particle binding with nanoMIP (25 µg mL−1). Scale bars, 200 nm (left) and 50 nm (right). b) Flow cytometry for the pseudovirus attachment to ACE2-HEK293T cells (C, control group; C + V, cells incubated with pseudovirus; C + V + MIP, cells incubated with pseudovirus pretreated with nanoMIP; 1 mg mL−1 nanoMIP was used). c) Confocal fluorescence images for the pseudovirus attachment to ACE2-HEK293T cells in the presence of nanoMIP (1 mg mL−1). Green represents the virions stained by DIO and blue represents the nucleus stained by DAPI. Scale bars, 20 µm (5 µm for enlarged images).
Hypervalent Glycan Shield-Binding Nanoparticles
In this study, molecularly imprinted nanoparticles were utilized to target the viral glycan shield by binding to it. The imprinted nanoparticles showed a broad spectrum of viral inhibition, targeting those carrying high mannose, particularly the wild-type SARS-CoV-2 variant.
Here, the ability of saccharide recognition based on boronate affinity was integrated with the controlled molecular imprinting technique to prepare nanoparticles, which could bind to high-mannose glycans in virion glycan shields.
Thus, the high binding capacity of the virion’s glycan shield enabled imprinted nanoparticles to block receptor-virus interactions. In addition, these nanoparticles exhibited a unique strength that was otherwise not provided by other low-valent neutralizing reagents.
Interestingly, multivalent synergetic binding of nanoparticles with high-mannose glycans enhanced their binding strength by two to three-fold compared to native mannose. Additionally, hypervalency of the nanoparticles induced viral aggregation, promoting viral clearance and blocking viral entry.
At the pseudovirus level, in addition to the antiviral effect against SARS-CoV-2 variant, the nanoparticles exhibited broad-spectrum antiviral efficacy against other variants such as N501Y, D614G, Δ69-70, N439K, omicron, and delta.
Notably, the high antiviral potency of imprinted nanoparticles was demonstrated by their EC50 value of approximately 109 molar units for all the tested viruses. Both delta and wild-type SARS-CoV-2 variants demonstrated a broad-spectrum neutralization efficacy against authentic viruses.
The unique double-punch mechanism and the hypervalency of mannose-binding nanoparticles offered an exceptional strategy for efficient and broad-spectrum inhibition of different species of viruses, turning the focus to glycan shielding and combating the issues related to diversity and mutation in viruses.
Thus, the present study, for the first time, has provided a virus inhibitor against a broad range of virus species, with significant prospects for expansion to inhibit the progression of newly emerging SARS-CoV-2 variants and other lethal viruses.
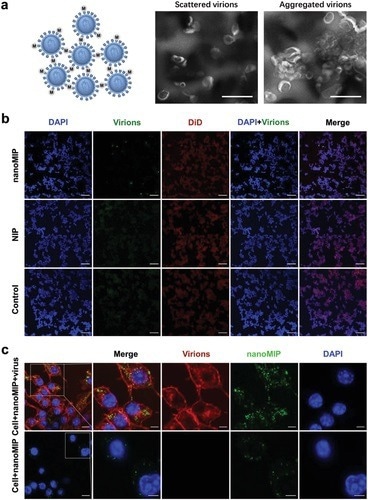
Figure 3. Viral aggregation induced by nanoMIP. a) Schematic of formation of virus aggregates induced by 200 µg mL−1 of nanoMIP (left). Negatively stained transmission electron microscopy images for scattered virions and aggregated virions induced by 200 µg mL−1 of nanoMIP (right). Scale bars, 500 nm. b) Representative confocal fluorescence images of SARS-CoV-2 pseudovirus treated with 200 µg mL−1 of nanoMIP, NIP, and nothing, respectively, in the presence of ACE2-HEK293T cells. Red represents the cell membrane stained by DID, green represents the virions stained by DIO and blue represents the nucleus stained by DAPI Scale bars, 100 µm. c) Confocal fluorescence images for macrophage uptake of nanoMIP and virus aggregates induced by nanoMIP (the middle image without ruler represents the lack of corresponding channel information, 200 µg mL−1 of nanoMIP was used). Red represents the virions stained by DID, green represents the FITC-doped nanoMIP, and blue represents the nucleus stained by DAPI. Scale bar: 10 µm (5 µm for enlarged images).
Conclusion
In summary, a hypervalent glycan-shield-targeting synthetic antibody was prepared with a rigid nanoscale structure for broad-spectrum antiviral activity. The synthesized nanoparticles exhibited hypervalent binding capacity with mannose.
The binding strength of the nanoparticles has increased by two to three orders when bound with virions modified with high-mannose glycans. The dissociation constant (Kd) value of imprinted nanoparticles was found to be 8.5 x 1010 molar units when used against the wild-type SARS-CoV-2 variant.
Furthermore, hypervalent mannose-binding nanoparticles and the unique double-punch mechanism have provided a distinctive approach for effective and broad-spectrum antiviral inhibition, shifting the focus from the changeable and mutating peptide epitopes in traditional techniques to glycan shields, thus, tackling the difficulties related to virus variability and mutation.
Therefore, the present work demonstrated a remarkable advantage and great promise of glycan-shield binding nanosized antibodies to combat COVID-19, along with broad-spectrum inhibition against other life-threatening viruses that may affect public health.
Reference
Li, Y., et al., (2022). Rational Development of Hypervalent Glycan Shield-Binding Nanoparticles with Broad-Spectrum Inhibition against Fatal Viruses Including SARS-CoV-2 Variants. Advanced Science. https://doi.org/10.1002/advs.202202689
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.