What if doctors could guide life-saving treatments through the body using nothing but a magnet?
An interdisciplinary team at the University of Pittsburgh’s Swanson School of Engineering is working to make that idea a reality. They've developed silk iron microparticles (SIMPs) - tiny, magnetic, biodegradable carriers designed to deliver drugs and treatments directly to specific sites in the body, such as aneurysms or tumors.
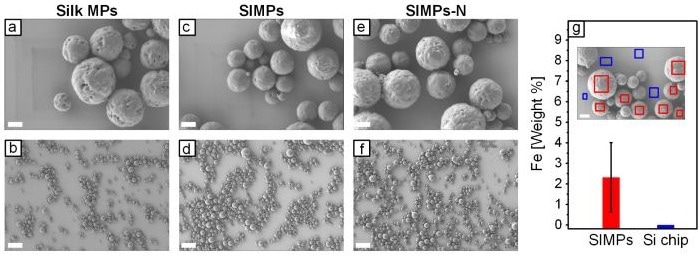 Scanning electron microscope (SEM) images of MPs. Representative (c) high and (d) low magnification images depict SIMPs with IONPs conjugated with GSH. Image Credit: University of Pittsburgh
Scanning electron microscope (SEM) images of MPs. Representative (c) high and (d) low magnification images depict SIMPs with IONPs conjugated with GSH. Image Credit: University of Pittsburgh
The effort is led by Pitt alumna Ande Marini (BioE PhD ‘25), now a postdoctoral scholar in cardiothoracic surgery at Stanford University, alongside David Vorp, John A. Swanson Professor of Bioengineering, and Justin Weinbaum, research assistant professor of bioengineering.
Their findings, titled “Chemical Conjugation of Iron Oxide Nanoparticles for the Development of Magnetically Directable Silk Particles” (DOI: 10.1021/acsami.4c17536), were published in the February edition of ACS Applied Materials & Interfaces.
Marini and her colleagues were motivated by their lab’s ongoing work to improve treatment options for abdominal aortic aneurysms (AAA), a condition that can be fatal if left untreated and contributes to nearly 10,000 deaths annually.
Their approach focuses on noninvasive, early-stage delivery of regenerative therapies using extracellular vesicles - small, membrane-bound capsules that help cells communicate.
We want to find a way to deliver extracellular vesicles to the site of an abdominal aortic aneurysm in the least invasive way possible. We envisioned that we could inject extracellular vesicles onto a carrier and then somehow guide the carrier to the outside of the aortic wall, so we came up with the idea of using magnetic attraction.
David A. Vorp, Department of Bioengineering, University of Pittsburgh
To bring that idea to life, the team partnered with Mostafa Bedewy, associate professor of mechanical engineering and materials science at the Swanson School, and his former Ph.D. student, Golnaz Tomaraei (IE PhD ‘23).
Their expertise in nanomaterials and fabrication was essential in producing the magnetic nanoparticles, each just one-hundred-thousandth the width of a human hair. At that scale, materials behave differently, taking on properties like magnetic responsiveness.
Our role was to synthesize magnetic nanoparticles with the right properties and bond them to the silk so they would stay attached during movement. You can think of it like towing cargo we created the particles to carry drugs, and the nanoparticles are the tow hook.
Mostafa Bedewy, Department of Mechanical Engineering and Materials Science, University of Pittsburgh
While magnetically guideable materials have been used in medical applications before, what sets this work apart is the chemical bonding of the magnetic particles to silk - a biocompatible material already approved by the FDA - using the compound glutathione.
We bridged biomaterials and chemical conjugation to create particles that could be magnetically guided. By chemically bonding iron oxide nanoparticles to the regenerated silk fibroin, we enhanced their magnetic movability so we can potentially localize them externally to a site of interest in the body.
Ande X. Marini, Department of Bioengineering, University of Pittsburgh
This breakthrough opens up a range of future possibilities, from targeted cancer treatments to regenerative therapies for cardiovascular disease. The immediate next step will be loading these carriers with therapeutic agents.
Marini said, “With this paper, we are showing that we can create an empty carrier that can be magnetically moved. The next step is figuring out what kind of cargo we can load, regenerative factors, drugs, or other materials people want to magnetically localize. Whether it is delivering cancer drugs with fewer side effects or slowing down tissue degradation in aneurysms, this technology has broad potential for regenerative medicine.”
For Bedewy’s team, this also means continuing to refine the molecular structure of the particles and optimizing how they release their cargo over time.
Bedewy said, “We are trying to create a toolbox of treatments, and in materials science, there is a lot of room for making more tools that can be useful for medical doctors and bioengineers to help create different ways of resolving problems in the body. This is an exciting project where people with very different sets of expertise came together to solve a problem and produce an outcome that could potentially have an immense impact on human lives.”
Journal Reference:
Marini, A. X., et al. (2025) Chemical Conjugation of Iron Oxide Nanoparticles for the Development of Magnetically Directable Silk Particles. ACS Applied Materials & Interfaces. doi.org/10.1021/acsami.4c17536.