Reviewed by Lexie CornerMay 21 2025
A collaborative research team from Empa, ETH Zurich, the Cantonal Hospital of St. Gallen, and Zhejiang University in China has developed innovative nanomedicines designed to safely and effectively treat inflammatory conditions that can arise during pregnancy.
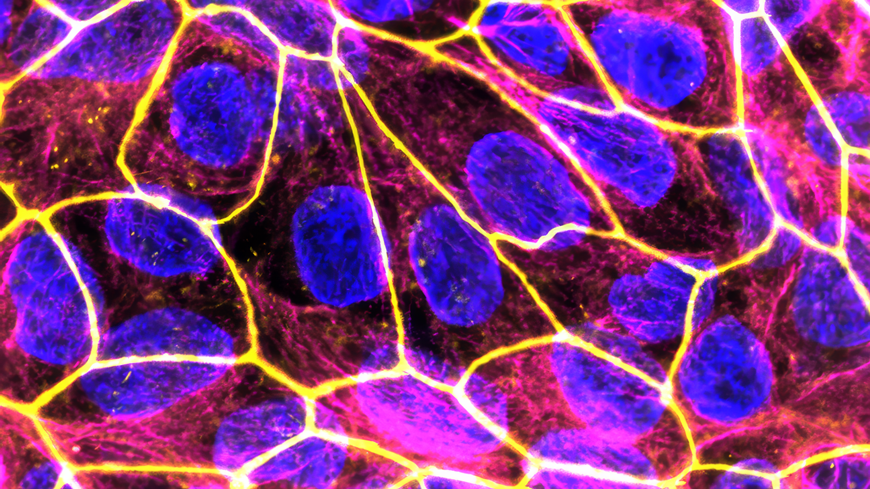 The new nanomedicines for the treatment of inflammatory processes during pregnancy should offer a safe therapy for both mother and child. The image shows the placenta model under the fluorescence microscope (blue: cell nuclei; yellow: cell-to-cell contacts). Image Credit: Empa
The new nanomedicines for the treatment of inflammatory processes during pregnancy should offer a safe therapy for both mother and child. The image shows the placenta model under the fluorescence microscope (blue: cell nuclei; yellow: cell-to-cell contacts). Image Credit: Empa
Treating illness during pregnancy requires particular caution, as many medications pose risks to both mother and fetus. Inflammation is a common factor in many pregnancy complications, yet current treatment options are often inadequate or raise concerns about their safety for fetal development.
This raises a critical question: what alternatives exist when standard medications for infections or pregnancy-related conditions, such as preeclampsia, gestational diabetes, or threatened preterm birth, are either ineffective or potentially harmful?
One promising avenue lies in nanozymes: tiny synthetic particles that may help manage inflammatory processes in the placenta without endangering maternal or fetal health. Researchers are rigorously evaluating the safety of these nanozyme-based therapies as part of a project supported by the Swiss National Science Foundation (SNSF).
A Modular Toolkit for Safe Therapies
Nanozymes are synthetic compounds on the nanometer scale that mimic the function of natural enzymes. Already being explored in areas like cancer therapy, they typically consist of a nanostructured core (such as metal atoms or metal oxides) that determines their enzymatic properties, along with surface modifications to enhance stability and target specificity.
In this way, we want to enable customized use for different areas of application.
Nikolaos Tagaras, Researcher, Empa
These nanozymes can adapt their activity based on the disease environment. Scientists can switch them from an inactive to an active state, allowing them to neutralize harmful reactive oxygen species (ROS) during inflammation or target and eliminate bacteria in cases of infection.
At Empa, the development of these nanozymes is accompanied by thorough safety testing. Researchers use established laboratory models that closely replicate the physiological conditions of the placenta and the maternal–fetal interface to assess how these particles behave in both the mother’s and the fetus’s systems.
The structure, metabolism, and interaction of maternal and fetal tissue are unique in humans.
Tina Bürki, Team Leader, Nanomaterials in Health Laboratory, Empa
It is therefore necessary to investigate the effect of the nanozymes using laboratory models based on human cells and tissues. For this purpose, the research team employs an established placental model using fully functional human placentas obtained following Caesarean sections.
“Only thanks to human placental tissue can we obtain meaningful results on the transport and effect of the nanozymes,” said the Empa researcher.
A Promising Start
Another step toward developing safe nanomedicines is the creation of a “placenta chip”—a finger-sized polymer device designed to support the growth of human cells that replicate the placental barrier and early embryonic tissue under near-physiological conditions. This innovative platform allows researchers to study both the transport of substances across the placenta and the direct and indirect effects of nanozymes on early embryonic development. Preliminary findings suggest promising results.
“The nanozymes do not impair the placental barrier and have so far shown no negative effects on the models studied,” says Empa researcher Tagaras.
Next, the team plans to investigate the anti-inflammatory and antibacterial properties of the nanozymes in greater detail.
Journal Reference:
Tagaras, N., et al. (2025) Safety Landscape of Therapeutic Nanozymes and Future Research Directions. Advanced Science. doi.org/10.1002/advs.202407816