Aug 22 2008
The electrical properties of all solid-state materials are governed by the way that electronic charge is distributed throughout their atomic structure. Now Kenichi Kato from the RIKEN SPring-8 Center, in Harima, and his colleagues have developed a technique for directly visualizing the distribution of charge within certain manganese oxides, which is a valuable tool for learning more about charge ordering in these and other materials*.
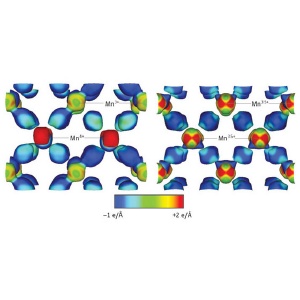 Image of the electrostatic potential on the electron density isosurface (contours in the atomic structure corresponding to a constant electron density of 0.8 e/Å3) of the ordered insulating state (left) and disordered metallic state (right) of the half-doped manganite, Nd1/2Sr1/2MnO3. Manganese (Mn) atoms bonded to neighboring oxygen atoms with a valency of 3+ and 4+ in the ordered state, and 3.5+ in the disordered state, are labeled.
Image of the electrostatic potential on the electron density isosurface (contours in the atomic structure corresponding to a constant electron density of 0.8 e/Å3) of the ordered insulating state (left) and disordered metallic state (right) of the half-doped manganite, Nd1/2Sr1/2MnO3. Manganese (Mn) atoms bonded to neighboring oxygen atoms with a valency of 3+ and 4+ in the ordered state, and 3.5+ in the disordered state, are labeled.
The charge ordering behavior of many rare-earth manganese oxide compounds holds a particular fascination for solid-state physicists. Under certain conditions of magnetic field and temperature, the electrons within such materials distribute themselves in unusual stripe-like patterns. This can dramatically change their magnetic and electronic properties. As well as being of fundamental interest, such effects could enable the development of sensitive sensors for high density magnetic storage applications. But the physical mechanisms responsible for this behavior are not yet understood completely.
To better study such phenomena, Kato and colleagues developed their technique for analyzing the way in which the distribution of charge within a material scatters synchrotron x-ray radiation. In doing so they have visualized the fine details of the charge distribution and electrostatic potential in an exotic electronic material known as half-doped manganite Nd1/2Sr1/2MnO3 and how these change with temperature.
At a temperature of 300 K (26.85 °C), the material exhibits a disordered charge distribution which gives rise to metallic electrical behavior. But at a much lower temperature of 18 K (-255.15 °C), the researchers observe that the charge distribution in certain planes of the material exhibits a zigzag pattern. More significantly, the amount of information that their technique provides enables them to determine exactly how the atoms within the structure of the material are bonded to each other under both conditions. In the disordered state, they find that the manganese atoms bond to neighboring oxygen atoms with a valency of +3.5. Whereas in the ordered state, they find they bond with a valency of either +3 or +4 depending on their position (Fig. 1).
The fine detail enabled by the technique even surprised the researchers. “At first we were not sure how clearly we would be able to observe the charge ordering state. So the level of clarity with which the technique showed up even very small variations in charge distribution was quite unexpected,” says Kato. “But the ability to observe how electrons behave in this way should enable materials scientists to design better materials for practical applications.”
*Kato, K., Moritomo, Y., Takata, M., Tanaka, H. & Hamada, N. Visualization of charge ordering in a half-doped manganite by an electrostatic potential analysis. Physical Review B 77, 081101R (2008).