Mar 18 2009
Johns Hopkins engineers have invented a method that could be used to help figure out how cancer cells break free from neighboring tissue, an "escape" that can spread the disease to other parts of the body. The new lab-on-a-chip, described in the March issue of the journal Nature Methods, could lead to better cancer therapies.
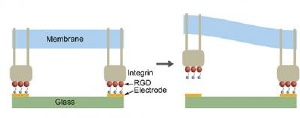 At left, the illustration shows adhesive complex formed between cell membrane-bound protein (integrin in gray) with peptide (RGD in red) tethered by thiol molecules (blue) to gold electrode (yellow) on glass slide (green). At right, electrochemical release of tethered peptide from the gold electrode results in local release of the RGD-integrin complex. Credit: Diagram by Peter Searson/JHU
At left, the illustration shows adhesive complex formed between cell membrane-bound protein (integrin in gray) with peptide (RGD in red) tethered by thiol molecules (blue) to gold electrode (yellow) on glass slide (green). At right, electrochemical release of tethered peptide from the gold electrode results in local release of the RGD-integrin complex. Credit: Diagram by Peter Searson/JHU
"Studying cell detachment at the subcellular level is critical to understanding the way cancer cells metastasize," says principal investigator Peter Searson, Reynolds Professor of Materials Science and Engineering. "Development of scientific methods to study cell detachment may guide us to prevent, limit or slow down the deadly spreading of cancer cells."
His team's research focuses on a missing puzzle piece in the common but unfortunate events that can occur in cancer patients. For example, cancer that starts in the breast sometimes spreads to the lungs.
That's because tumor cells detach and travel through the bloodstream to settle in other tissues. Scientists have learned much about how cancer cells attach to these surfaces, but they know little about how these insidious cells detach because no one had created a simple way to study the process.
Searson and two other scientists from Johns Hopkins' Whiting School of Engineering have solved this problem with a lab-on-a-chip device that can help researchers study cell detachment. With this device, they hope to discover exactly how cancer cells spread.
The lab-on-a-chip device consists of an array of gold lines on a glass slide. Molecules promoting the formation of cell attachments are tethered to the gold lines like balloons tied to string. A cell is placed on the chip, atop these molecules. The cell spreads across several of the gold lines, forming attachments to the surface of the chip with help from the molecules.
Then, the tethered molecules are released from one of the lines by a chemical reaction, specifically by "electrochemical reduction," Searson explains. Where these molecules are detached, that portion of the cell loses its grip on the surface of the chip. This segment of the cell pauses for a moment and then contracts forcefully toward its other end, which is still attached to the chip. The researchers were able to film this "tail snap" under a microscope.
"It's very dramatic," says Denis Wirtz, a Johns Hopkins professor of chemical and biomolecular engineering and co-author of the Nature Methods paper. "The cell stretches way, way out across the chip and then, on command, the tail snaps toward the body of the cell."
Cells survive this programmed-release process and can be tested again and again, the researchers said.
Bridget Wildt, a materials science and engineering doctoral student in Searson's lab, used the device to perform and record movies of the live-cell experiments. Wildt tested cells from the connective tissue of mice during these experiments, but the team plans to try other types of cells in the future.
"In the movies, you can see that the cell doesn't move immediately after the chemical reaction is triggered. We refer to this phenomenon as the induction time of the cell," Wildt says. "After this induction time, the cell then snaps back and contracts. We analyze the rate of the cell's contraction and then compare this information to separate cells released under different conditions using chemicals called inhibitors. From these results we are beginning to understand the processes that regulate cell detachment at the molecular level."
The researchers have speculated that the induction time for cancer cells, as compared to noncancerous cells, would be shorter because cancer cells are more pliable. In the near future, Wildt says, they plan to test this hypothesis in experiments with cancer cells. If this assumption proves correct, it may give them a tool to differentiate between cancerous and noncancerous cells.