Aug 14 2009
Friction limits the speed and efficiency of macroscopic engines. Is this also true for nanomachines? A Dresden research team used laser tweezers to measure the friction between a single motor protein molecule and its track. The team found that also within our cells, motors work against the resistance of friction and are restrained in its operation-usually by far not as much though as their macroscopic counterparts. These first experimental measurements of protein friction could help researchers to better understand key cellular processes such as cell division which is driven by such molecular machines. (Science, August 14, 2009)
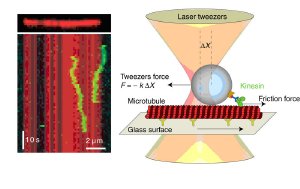 Abb.: Fluorescent image of single motor proteins (left): Motion of two diffusing kinesin molecules (green) on a microtubule (red) shown as a time series kymograph. Schematic (right): By dragging diffusing kinesin molecules with laser tweezers over a microtubule, the friction force between the motor and its microtubule track can be measured very precisely. Image: MPI-CBG, BIOTEC
Abb.: Fluorescent image of single motor proteins (left): Motion of two diffusing kinesin molecules (green) on a microtubule (red) shown as a time series kymograph. Schematic (right): By dragging diffusing kinesin molecules with laser tweezers over a microtubule, the friction force between the motor and its microtubule track can be measured very precisely. Image: MPI-CBG, BIOTEC
Friction is the force that resists the relative motion of two bodies in contact. The same is true on the nanoscale: Molecular motors have to fight the friction created between them and their tracks. However, since the frictional forces acting on such motors had not been measured before, it was not known how they depend on the speed and the direction of motion.
Friction Slows Down Proteins
Scientists in Dresden at the Biotechnology Center (BIO-TEC) of the Technical University of Dresden and at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) immobilized the molecular motor kinesin on a microsphere which was held by laser tweezers and dragged over its track, a so-called microtubule. In this manner, the friction force between the motor and its microtubule track was measured very precisely. "Just like for macroscopic machines, protein friction limits the speed and efficiency of the small bio-motors", says Erik Schäffer, group leader at the BIOTEC and Jonathon Howard, director and group leader at the MPI-CBG.
The researchers explain that the protein, in the absence of an energy source, takes eight nanometer (a millionth of a millimeter) wide "diffusive hops", corresponding to the length of the tubulin subunits that make up a microtubule. The motors step from one tubulin subunit to the adjacent one by forming a new bond with the microtubule filament as another bond is broken. When pulled by the tweezers, the energy released from these breaking bonds is lost as friction.
Efficient nanomachines
Protein friction also gives insight into the efficiency of kinesin. "About half of the energy from the motor’s fuel ATP is dissipated as friction between the motor and its substrate" Howard comments. Schäffer adds: "What remains after further dissipation inside the motor is used for mechanical work—the efficiency is usually much better than for man-made machines". The dissipated energy is eventually converted to heat, that contributes to the heating of our body. Thus, for example our muscles are partly heated by protein friction as the muscle motor proteins do their work.
Original work:
Volker Bormuth, Vladimir Varga, Jonathon Howard, Erik Schäffer
Protein friction limits diffusive and directed movements of kinesin motors on microtubules
Science 325, 870 (August 14, 2009) doi:10.1126/science.1174923