Oct 26 2010
Proteins are an interesting class of drugs because they demonstrate high biological activity and are highly specific in their effects. It has become possible to produce more and more proteins with tailored pharmacological properties; however transport and controlled release of the protein drugs in the body have remained a challenge.
In the journal Angewandte Chemie, Helmuth Mohwald of the Max Planck Institute of Colloids and Interfaces in Golm/Potsdam (Germany) and Dmitry V. Volodkin and Regine von Klitzing of the TU Berlin (Germany) have now introduced an alternative to the usual transport agents, such as liposomes: by using a simple, inexpensive, gentle process, they were able to produce pure protein microspheres of uniform size.
Loading nano- and microscale transport systems with proteins is the most common strategy used to bring drugs to their target area and achieve a longer period of activity. The challenge is to produce particles with a precisely defined quantity of protein, size, morphology, composition, and density. These characteristics are critical for the attainment of high bioavailability and a defined rate of release at the desired location. Unfortunately, they are difficult to control when using conventional methods for the production of protein particles, such as crystallization, spray drying, or incorporation in liposomes or polymer matrices. Another disadvantage is that these processes generally require organic solvents, high temperatures, or other conditions that can compromise the stability of the proteins.
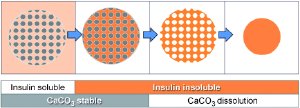
The researchers were looking for a method that would deliver uniform protein particles without destructive additives and under mild conditions. The team has now developed such a method, which is also very simple and inexpensive, and successfully tested it on insulin, a classic therapeutic protein. The secret to their success lies in porous calcium carbonate microspheres of defined size, and a change of pH value. In a slightly alkaline aqueous environment (high pH value), the protein insulin is soluble.
When the calcium carbonate spheres are added to such a protein solution, their pores are filled with the insulin solution. When the solution is then neutralized with acid, the insulin becomes insoluble and precipitates out in the pores. If the solution is acidified further, until the calcium carbonate spheres slowly begin to dissolve in the slightly acidic solution. The insulin remains behind as a loose matrix, which shrinks down into compact micrometer-scale spheres. This results in protein particles of uniform size and high protein density.