Nov 30 2017
Germanium nanoparticles with enhanced photoluminescence have been developed by researchers at the U.S. Department of Energy’s Ames Laboratory, making them potentially promising materials for solar cells and imaging probes. The researchers discovered that by adding tin to the nanoparticle’s germanium core, its lattice structure matched the lattice structure of the cadmium-sulfide coating in a better manner, thus allowing the particles to absorb more light.
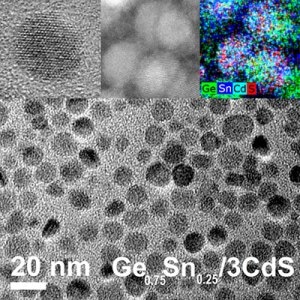 High-resolution and elemental mapping electron micrographs of near-infrared active GeSn/CdS nanocrystals. Credit: Ames Laboratory
High-resolution and elemental mapping electron micrographs of near-infrared active GeSn/CdS nanocrystals. Credit: Ames Laboratory
For a photovoltaic material, obviously absorbing light is the first part and converting that solar energy into electrical energy is the second part,So you want a material that does both efficiently. Germanium has some desirable characteristics for photovoltaic materials, but unfortunately it doesn’t absorb light well.
Emily Smith, Ames Laboratory scientist
Part of the problem exists in the fact that the outside surface of germanium nanoparticles varies over time, chiefly from oxidation. Earlier work by Ames Laboratory scientist Javier Vela’s group discovered that coating nanoparticles — commonly known as surface passivation — enhanced the nanoparticles’ potential to absorb light.
“We aren’t actually measuring absorption,” Smith explained, “we measure the luminescence – the amount light given off after a photon is absorbed. ”
“The fact that germanium doesn’t absorb light well is a simple way of saying it’s an indirect bandgap material,” Smith added, “and we are trying to make a more direct bandgap material, one that absorbs light better.”
The research literature suggests that the addition of tin appears to enhance germanium’s light absorption properties. But, the Ames Laboratory researchers managed to find that even with adding tin, the nanoparticles still need a surface coating. However, they also learnt that the relationship between the core material and the atomic structure of surface coating can further improve the light absorption.
The particular method employed is known as Successive Ion Layer Adsorption and Reaction (SILAR), which was primarily adapted in order to group IV colloids a number of years ago.
"We have been developing the expertise required to grow intricate core/shell and other well defined nanoparticles for many years," Vela said, “Through our collaboration with Emily Smith’s group, we hope to continue making inroads in our ability to manipulate and direct energy flows at the nanoscale."
The researchers used powder X-ray diffraction and transmission electron microscopy imaging to study the Raman and photoluminescence spectroscopies and structural characteristics of the nanoparticles in order to quantify photoluminescence behavior and lattice strain, and they discovered a correlation between the quantity of tin in the core and how well the lattice of the core matched that of the cadmium-sulfide outer shell.
The atoms are in a very specific location within the nanocrystal core and when you apply the shell around the nanocrystal, the atoms of the shell may not match perfectly with the atoms of the core, with the germanium only material used previously, the core and shell didn’t match perfectly. When we studied the germanium-tin particles, we proposed that they worked better because the spacing of the atoms better matches the spacing of the atoms we used in the coat layer, by doing that, you’re getting a more perfect shell that’s less likely to cause chemical changes to the surface of the nanoparticle core.
Emily Smith, Ames Laboratory scientist
Another promising use for this material, besides photovoltaics, can be found in imaging or microscopy, researchers frequently need to “label” a protein or any other feature with a nanoparticle “probe” in order to make it light up so that it is easier to observe and study.
The research results, “Germanium-Tin/Cadmium Sulfide Core/Shell Nanocrystals with Enhanced Near-Infrared Photoluminescence,” were featured in the American Chemical Society’s journal Chemistry of Materials.