Jan 5 2018
Artificial polyelectrolytes can imitate interactions of biological macromolecules like proteins, nucleic acids, and polysaccharide–protein conjugates. Such synthetic polyionic complexes are expected to function as unique platforms for stabilizing and delivering proteins, drugs, or nucleic acids.
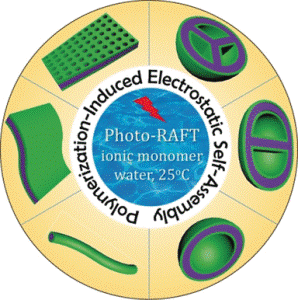 Photo credit: © Wiley-VCH
Photo credit: © Wiley-VCH
Chinese investigators have reported a versatile, commercially applicable approach of such nanomaterials with tunable morphology in the Angewandte Chemie journal. It is possible to envisage the preparation of libraries of these low-dimensional biorelevant nanostructures.
Many polysaccharide–protein conjugates, proteins, DNA and RNA are charged biological macromolecules. They comprise of complex structures along with unique functions, bringing about the possibility of cellular life. Not surprisingly, synthetic polyionic assemblies that imitate the properties of the biological macromolecules are expected to work as perfect platforms for interaction with biology. Such polyion complexes or PICs, with their controllable shape and charge state, could act as active carriers for nucleic acids in gene therapy and also for the targeted drug delivery. However, the rational design of the PICs continues to be challenging: structure, charge state, and final morphology depend on thousands of thermodynamic and kinetic parameters. Shape, stability and reactivity are often not reproducible. At Soochow University in Suzhou, China, investigator Yuanli Cai and his colleagues are thus enhancing rationalized preparation schemes. With the method known as "polymerization-induced electrostatic self-assembly" (PIESA), they have presently projected a cost-effective and scalable preparation protocol for low-dimensional PICs with tunable morphologies for biomedical use.
The protocol has been based on the polymerization-induced self-assembly method (PISA) in order to reasonably synthesize block copolymer nanoparticles in aqueous medium. The authors expanded the protocol by launching a positively charged monomer, and this was then polymerized in the presence of a pre-synthesized polyion of opposite charge and another macromolecule serving as an uncharged copolymer block. The final nanomaterial was made up of defined complexes of the charged copolymers and polymers. It showed incredible properties.
Dependent on the concentration of solids, the authors noticed structural transitions of the synthesized PICs from vesicles to compartmented vesicles to big-area ultrathin flexible films. Furthermore, dependent on the solvent used, either pore-dense films or very long nanowires became dominant, the latter resulting to gelation. The authors highlighted that their PIESA protocol under polymerization with visible-light produces “high structure reproducibility on a commercially viable scale under eco-friendly aqueous conditions at 25 °C.” This means that complex nanomaterials with tunable morphology and charge state could be effortlessly prepared. Biomedical applications for carrying and delivering of DNA and various other biological charged polymers at their site of action are visualized, including a library of low-dimensional nanomaterials with tunable morphology.