Feb 14 2018
You cannot look deep into a well or under the ocean’s surface as it gets dark because light cannot penetrate so far down. Despite the fact that human brain is not bottomless, neuroscientists experience the same deficiency of light while attempting to investigate living deep-brain structures.
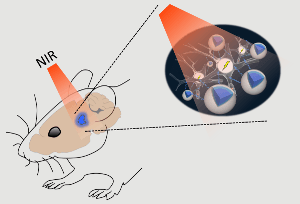 Schematic of the stimulation process. Near-infrared (NIR) light applied above the skull can quickly pass through brain tissue with minimal scattering and reach deep structures. Up-conversion nanoparticles (UCNPs; blue) in the tissue can absorb this light and locally emit visible light sufficient to activate light-sensitive channels expressed in nearby neurons. (Image credit: RIKEN Brain Science Institute)
Schematic of the stimulation process. Near-infrared (NIR) light applied above the skull can quickly pass through brain tissue with minimal scattering and reach deep structures. Up-conversion nanoparticles (UCNPs; blue) in the tissue can absorb this light and locally emit visible light sufficient to activate light-sensitive channels expressed in nearby neurons. (Image credit: RIKEN Brain Science Institute)
This problem is frustrating because optogenetics — a technique for controlling genetically tagged brain cells by using light — has become highly popular in the last ten years.
Optogenetics has been a revolutionary tool for controlling neurons in the lab, and hopefully someday in the clinic. Unfortunately, delivering light within brain tissue requires invasive optical fibers.
Thomas McHugh - Lead Researcher, RIKEN Brain Science Institute, Japan
At present, McHugh and his team have a solution for directing light to new depths in the brain. As described in the Science journal on February 9, 2018, upconversion nanoparticles (UCNPs) can function as a conduit for the delivery of laser light from outer side to the skull. Such nanoparticles can absorb near-infrared laser light and in turn discharge visible photons to regions inaccessible to standard optogenetics. This technique was adopted to turn on neurons in different regions of the brain areas and also to suppress seizure activity and stimulate memory cells.
“Nanoparticles effectively extend the reach of our lasers, enabling the ‘remote’ delivery of light and potentially leading to non-invasive therapies,” stated McHugh.
In the case of optogenetics, blue-green light is used to turn on or off neurons through light-responsive ion channels. However, light at such wavelengths gets scattered powerfully and reaches the other end of the spectrum from the near-infrared light that can travel deeper into brain tissue. UCNPs formed of elements belonging to the lanthanide family have the potential to function as a bridge. Their “optogenetic actuation” transforms low-energy near-infrared laser light into green or blue wavelengths for regulating particular labeled cells. Despite the fact that such light bursts impart a significant amount of energy to a small region, increases in temperature or damage of cells were not noticed.
Apart from activating neurons, UCNPs can also be applied for inhibition, for instance, to suppress experimental seizures in mouse models. The team administered nanoparticles adapted to discharge green light into the hippocampus and then laser pulses were used to excite them at the surface of the skull. Hyperexcitable neurons were efficaciously silenced in the mice.
In the medial septum, another region of the brain, nanoparticle-emitted light acted to synchronize neurons in a significant brain wave known as the theta cycle. In the case of mice that had imparted fear memories, the freezing behavior linked to such experiences was stimulated by blue light-emitting UCNPs, again in the hippocampus. Neural activation, inhibition, and memory recall impacts were noticed only in mice that were administered with nanoparticle-mediated optogenetic stimulation, but not in control mice that were administered with laser light sans a UCNP injection.
Memory recall was also observed to persist in investigations performed two weeks later. This suggests that the UCNPs stayed at the injection site, which was asserted by performing microscopy of the brains.
“The nanoparticles appear to be quite stable and biocompatible, making them viable for long-term use. Plus, the low dispersion means we can target neurons very specifically,” stated McHugh.
The nanoparticles reported in this research are well-suited to the different light-stimulated channels prevalently used in the optogenetics field and can be used for neural stimulation or inhibition in various deep brain structures. Nanoparticles can emerge as a minimally invasive substitute for optical fibers for brain activation, and their chronic interaction with brain tissue is part of ongoing research.
This research was the result of a partnership between researchers at the RIKEN Brain Science Institute, the National University of Singapore, the University of Tokyo, Johns Hopkins University, and Keio University.
Reference
- Shuo Chen,Adam Z Weitemier,Xiao Zeng, Linmeng He, Xiyu Wang, Yanqiu Tao, Arthur JY Huang, Yuki Hashimotodani, Masanobu Kano, Hirohide Iwasaki, Laxmi Kumar Parajuli, Shigeo Okabe, Daniel B Loong Teh, Angelo H All, Iku Tsutsui-Kimura, Kenji F Tanaka, Xiaogang Liu and Thomas J McHugh, "Near-infrared Deep Brain Stimulation via Upconversion Nanoparticle-mediated Optogenetics.", Science, doi: 10.1126/science.aaq1144