By Matt Pullen, B.Sc.Feb 17 2020
Since its discovery, copper has been vital to human technology. In ancient times, it was used to make weapons and tools. Even today, it finds extensive use, specifically in electronic devices that need wiring.
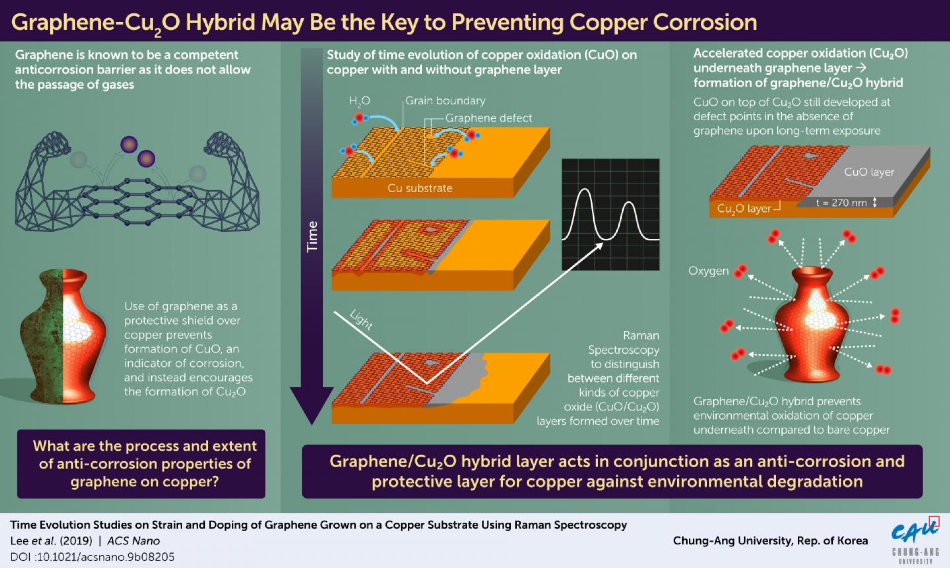 Scientists in Korea are first to observe an unprecedented way in which graphene forms a hybrid layer that prevents copper corrosion. Image Credit: Chung-Ang University.
Scientists in Korea are first to observe an unprecedented way in which graphene forms a hybrid layer that prevents copper corrosion. Image Credit: Chung-Ang University.
However, a difficulty faced while using copper is the oxidization of its surface over time, even under ambient conditions, eventually resulting in corrosion. Therefore, formulating a long-term technique to safeguard the exposed surfaces of copper is an important goal.
Coating the surfaces of metal with anti-corrosive substances is one common way to prevent corrosion. Graphene has been widely investigated as a substance for anti-corrosive coating because it acts as a barrier to gas molecules.
However, in spite of these characteristics, graphene sheets have been observed to protect copper from corrosion only for shorter periods of time (less than 24 hours). An astonishing fact is that following this initial period, graphene seems to increase the rate of corrosion of copper, which is completely contradictory to its anti-corrosive property.
Led by Prof. Hyungbin Son, a team of researchers from Chung-Ang University, Korea, analyzed graphene islands on a copper substrate to study its corrosion patterns in order to gain more insights into the unique nature of graphene observed in its interactions with copper.
Graphene is known to be mechanically very strong and impermeable to all gases, including hydrogen. Following studies claiming that the corrosion of copper substrates was accelerated under graphene through various defects, these properties have attracted great attention as an oxidation barrier for metals and have been controversial for over a decade.
Hyungbin Son, Professor, Chung-Ang University
Prof. Son continued, “However, they have not been qualitatively investigated over longer time scales. Thus, we were motivated to study the role of graphene as a corrosion-resistant film at the graphene-copper interface.”
Raman spectroscopy, white light interferometry, and scanning electron microscopy were used by Prof. Son and his colleagues to look at the trends in copper corrosion for 30 days.
Initially, the researchers identified that corrosion developed at the edges, spreading copper oxide (Cu2O), the oxidized form of copper, at different defects like grain boundaries, edges, and missing atoms. This led to the splitting of water vapor, which supplied oxygen for the oxidation process, to a point where the entire barrier appeared to be rendered useless and copper was completely corroded beneath.
Due to graphene’s impact on ambient water vapor, the copper substrate’s protected portion was highly corroded compared to the unprotected portion. As time passed, the build-up of Cu2O beneath the graphene sheet scattered the strain and led to p-doping in graphene—forming a hybrid-like structure. However, following 13 days of exposure to ambient conditions, the researchers found something new.
They noticed that the corrosion had considerably slowed down, with the formation of a new hybrid of graphene and Cu2O layer. At the same time, continuous corrosion of the unprotected copper was observed at a constant rate, until it had infiltrated far deeper compared to the corrosion beneath the graphene shield.
These outcomes reveal that graphene, indeed, serves to protect copper from deep, penetrating oxidation, in contrast to the conclusions of earlier studies.
We observed that over a longer time scale (more than 1 year), the graphene-Cu2O hybrid structure became a protective layer against oxidation. The area beyond the graphene was heavily oxidized with CuO, with a depth of 270 nm.
Hyungbin Son, Professor, Chung-Ang University
Finally, this research has found a solution to the question of whether it is possible to use graphene to protect copper against oxidation.
For nearly a decade, graphene’s anti-corrosive properties have been controversial, with many studies suggesting that graphene accelerates the oxidation of copper (resulting in its corrosion). We have shown for the first time that the graphene-Cu2O hybrid structure, which forms over a long period, significantly slows down the oxidation of copper in the long term, as compared to bare copper.
Hyungbin Son, Professor, Chung-Ang University
More time will be needed before establishing further applications of graphene as an anti-corrosive material. However, one definite thing is that this study has prospectively brought down various barriers in using graphene to increase copper’s service life.