Carbon displays an extraordinary tendency to produce nanomaterials with rare chemical and physical properties, owing to its ability to participate in diverse bonding states.
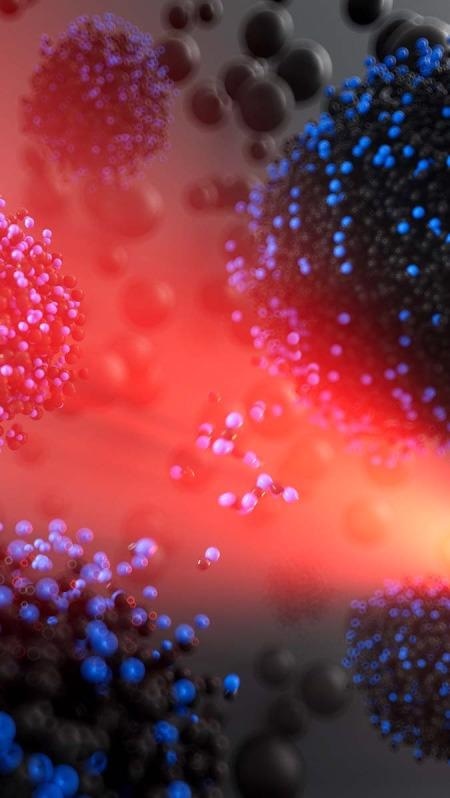 Artist’s interpretation of reactive transport between liquid nanocarbon clusters predicted to form from shock-compressed cryogenic liquid carbon monoxide. Small black and blue beads correspond to carbon and oxygen atoms respectively, and the red light is meant to evoke the laser used to drive shock compression experiments. Image Credit: Brendan Thompson/LLNL
Artist’s interpretation of reactive transport between liquid nanocarbon clusters predicted to form from shock-compressed cryogenic liquid carbon monoxide. Small black and blue beads correspond to carbon and oxygen atoms respectively, and the red light is meant to evoke the laser used to drive shock compression experiments. Image Credit: Brendan Thompson/LLNL
Several of these “next-generation” nanomaterials, which comprise nanographite, nanodiamonds, nano-onions and amorphous nanocarbon, are presently being examined for potential applications encompassing fields from bio-imaging to quantum computing. Continuing research proposes that high-pressure synthesis employing carbon-rich organic precursors could pave the way for the discovery and perhaps the custom-made design of many more.
Researchers from Lawrence Livermore National Laboratory (LLNL) were keen to better comprehend how to custom-make carbon nanomaterials and how their development affects shock phenomena like detonation.
Therefore, the LLNL team carried out machine-learning-stimulated atomistic simulations to offer insight into the important processes regulating the development of nanocarbon materials, which could function as a design instrument, help direct experimental efforts and facilitate more precise energetic materials modeling.
Laser-stimulated shock and detonation experiments can be employed to push carbon-abundant materials to conditions of pressures of 10s of GPa (one GPa equals 9,869 atmospheres) and temperatures of the 1,000s of degrees Kelvin (K), under which multifaceted processes lead to the development of 2-10 nanometer nanocarbons in a matter of 100s of nanoseconds.
However, the accurate physical and chemical phenomena controlling emergent nanocarbon formation under very high temperature and pressure have not been completely investigated yet, partly because of the issues related to examining systems at such extreme states.
Latest experiments on nanodiamond manufacture from hydrocarbons exposed to conditions akin to those of planetary interiors provide certain clues on probable carbon condensation mechanisms, but the landscape of conditions and systems under which strong compression could produce remarkable nanomaterials is too vast to be investigated using only experiments.
The researchers learned that liquid nanocarbon development follows traditional growth kinetics stimulated by Ostwald ripening (evolution of large clusters at the cost of shrinking small ones) and conforms to dynamical scaling in a process facilitated by reactive carbon transport in the immediate fluid.
The results provide direct insight into carbon condensation in a representative system and pave the way for its exploration in higher complexity organic materials, including explosives.
Rebecca Lindsey, Study Co-Lead Author and Researcher, LLNL
The corresponding research article has been published in Nature Communications.
The modeling effort of the researchers included a detailed investigation of carbon condensation (precipitation) in oxygen-deficient carbon oxide (C/O) mixtures at high temperatures and pressures, rendered feasible by mass replications using machine-learned interatomic potentials.
Carbon condensation in organic systems exposed to high pressures and temperatures is a non-equilibrium procedure similar to phase separation in mixtures quenched from a homogenous phase into a two-phase region, however, this association has only been partly investigated; particularly, phase separation theories remain highly applicable for nanoparticle synthesis.
The LLNL team's simulations of chemistry-coupled carbon condensation and associated analysis look into longstanding queries associated with high-pressure nanocarbon production in organic systems.
Our simulations have yielded a comprehensive picture of carbon cluster evolution in carbon-rich systems at extreme conditions—which is surprisingly similar with canonical phase separation in fluid mixtures—but also exhibit unique features typical of reactive systems.
Sorin Bastea, Study Principal Investigator, Study Co-Lead Author, and Physicist, LLNL
Other LLNL researchers involved in the study include Laurence Fried and Nir Goldman. LLNL’s Laboratory Directed Research and Development program funded the study.
Journal Reference:
Lindsey, R.K., et al. (2022) Chemistry-mediated Ostwald ripening in carbon-rich C/O systems at extreme conditions. Nature Communications. doi.org/10.1038/s41467-022-29024-x.