For this reason, several experiments were designed to synthesize citrate-capped spherical AuNPs and investigate the effects of a broad range of initial pH values, trisodium citrate and chloroauric acid (Na3Cit/HAuCl4) molar ratios, reaction temperature, and the two-order reagent addition methods, method I and method II, on the ultimate reaction efficacy and characterizations. The characterization of prepared nanoparticles (NPs) synthesized through various research included dynamic light scattering, Fourier transform infrared spectroscopy and UV-Visible.
Additionally, NPs made under ideal synthesis circumstances were examined in greater detail with X-ray diffraction (XRD), transmission electron microscopy, and UV-Visible. The results showed that all examined synthesis parameters and the order of reagent addition significantly affected the ultimate size and synthesis efficiency of citrate-capped spherical AuNPs. Also, the increased Na3Cit/HAuCl4 Molar ratio and the initial reaction temperature achieved a smaller particle size with desired synthesis efficiency.
Likewise, cubic crystal structures of the ultimate optimized NPs were presented, and a single crystal of each NP was obtained. In conclusion, this research showed that optimizing synthesis conditions enhanced the final NPs’ efficiency, which was a key component of future cost-effectiveness. It also improved the morphology, size distribution, and crystallite size of the final NPs.
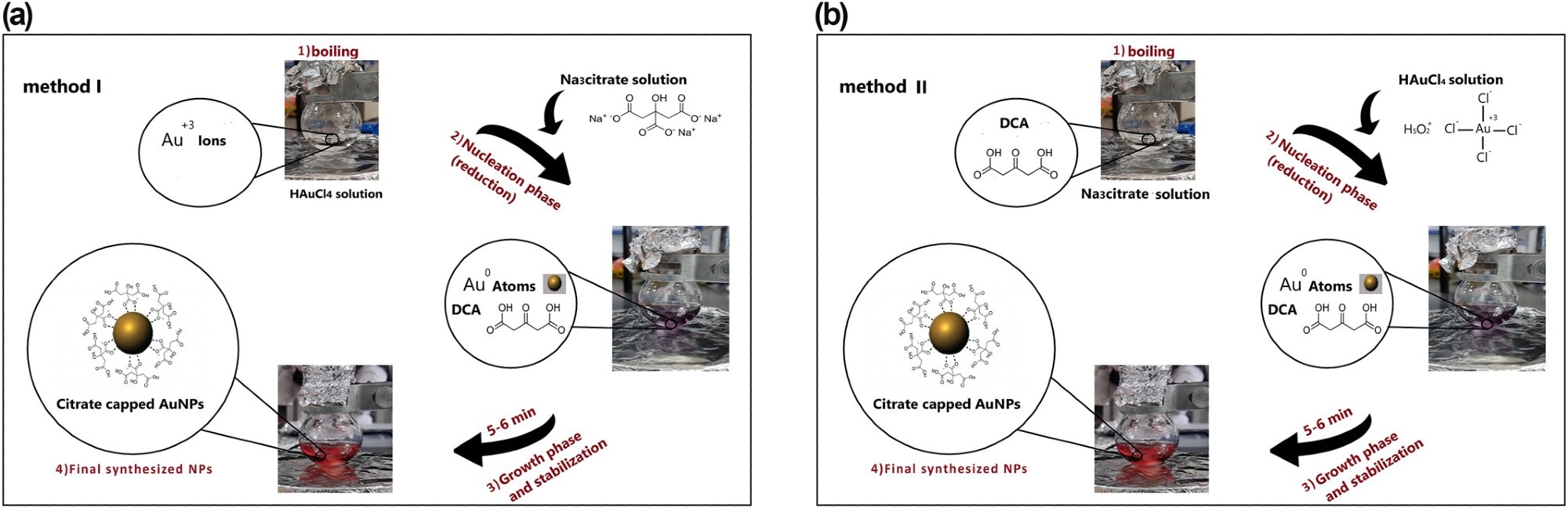
Figure 1. Schematic view for the fabrication of spherical citrate-capped gold nanoparticles (AuNPs), based on different reagent addition orders of (a) Turkevich approach as method I and (b) Reverse of Turkevich approach as method II. © Bahmanyar, Z., et al. (2022).
Understanding and Developing the Potential of AuNPs
AuNPs have recently gained considerable biomedical interest due to their excellent biocompatibility, desirable physicochemical qualities, and adaptability of characteristics in synthesis. Numerous biomedical uses, such as gene and drug transport, biosensors, hyperthermia and phototherapy, and antimicrobial applications in various shapes of nanorods, nanostars, nanocages, and nanospheres, have all been researched using gold nanoparticles.
It is commonly acknowledged that the NPs’ intrinsic actions in in vivo and in in vitro applications are determined by their physicochemical characteristics, particularly their morphology and size. According to earlier research, AuNPs between 30 and 10 nanometers in size are implanted more readily into malignant tumor cells than bigger ones. Smaller diameters of about 20 nanometers of surface-coated AuNPs demonstrated greater cell uptake than the AuNPs between 40 and 80 nanometers.
Though smaller AuNP sizes are frequently utilized in various applications, there may be some possible disadvantages. Due to their chemical reactivity, the NPs’ minimum size, under five nanometers, is observed to have more significant toxicity. Additionally, earlier research indicated that the spherical AuNPs having a size of about 1.4 nanometers, caused mitochondrial damage, oxidative stress, and necrosis in the cell lines under investigation.
Since AuNPs’ toxicity is size-dependent, preparing them in the ideal and acceptable size for every application type is crucial to minimize negative consequences. Here the authors constructed several synthesis experiments to optimize the manufacture of AuNPs with desired sizes of nanospheres and appropriate synthesis productivity for biological applications.
Thus, various experiments were created utilizing the Turkevich method by varying the reaction conditions, such as the reaction's initial temperature, PH, and the spectrum of the Na3Cit/HAuCl4 molar ratios.
The Turkevich method was a reproducible and convenient technique that used Na3Cit as a stabilizing and reducing agent to produce small-sized gold nanospheres. It reduced gold chloride salt in an aqueous solution to produce monodisperse AuNPs suspensions with variable particle sizes. Also, the designed synthesis investigations were performed in two distinct reagent addition orders, including Na3Cit salt and HAuCl4, considering the significant influence of the added orders of precursors on the properties of final particles and synthesis performance.
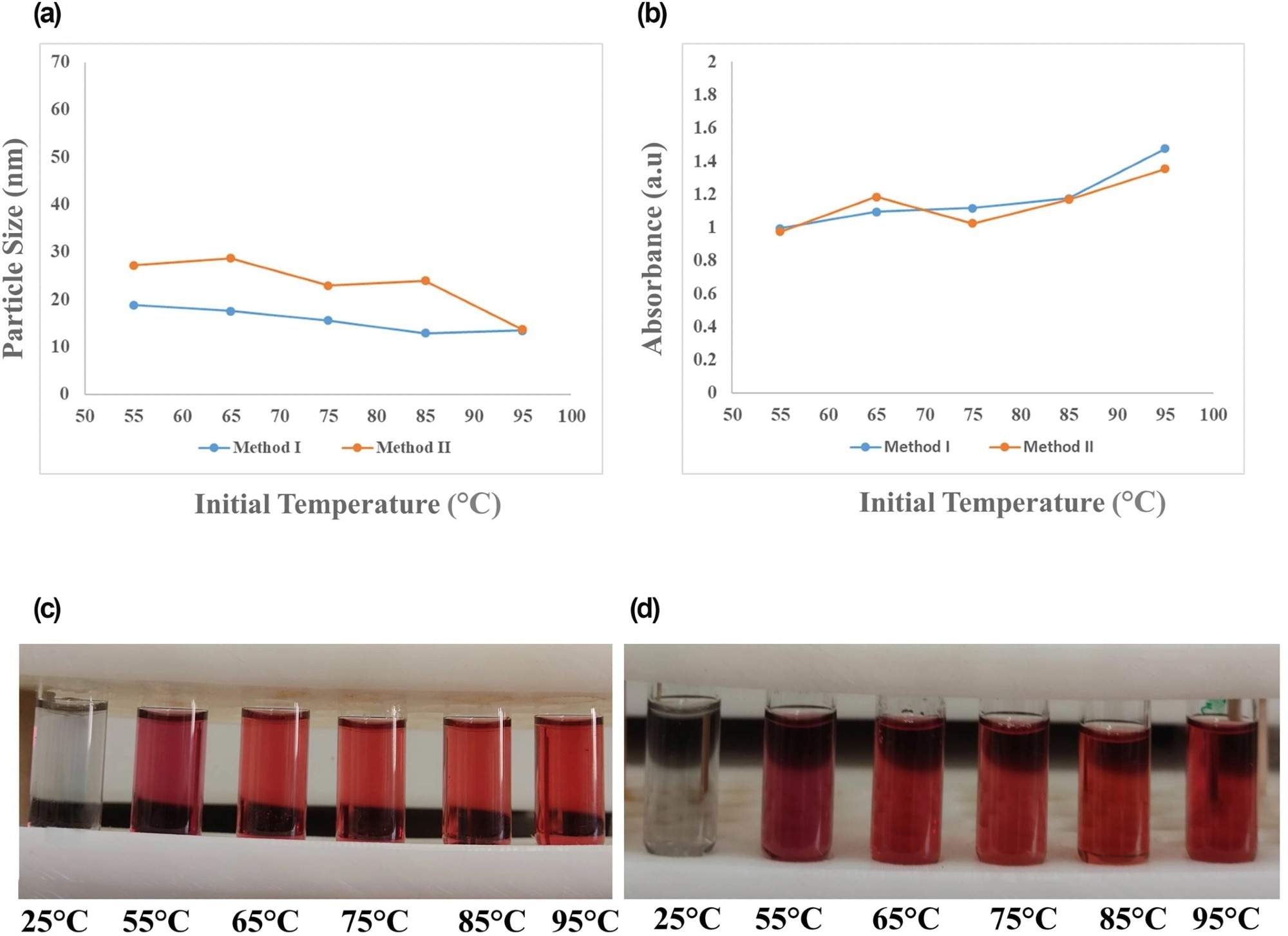
Figure 2. Effect of initial reaction temperature of 25, 55, 65, 75, 85, and 95 °C on (a) final particle size and (b) maximum SPR absorption of gold nanoparticles (AuNPs) synthesised by two methods, along with the laboratory images of NPs obtained by (c) method I and (d) method II. SPR, surface plasmon resonance. © Bahmanyar, Z., et al. (2022).
Proof-of-Concept Investigations
The round-bottom flask was rinsed with freshly made aqua regia acid solution before each step of the synthesis process to avoid contamination. 58 microliter of 0.05 Molar HAuCl4 were mixed with 10 milliliters of deionized water. The mixture was aggressively stirred while the precise Na3Cit solution concentrations were introduced abruptly. Before adding the reducing agent, constant air pressure was required.
The synthesis investigations were conducted using two different methods. Method I involved adding a specific concentration of Na3Cit solution to a boiling gold salt solution. At the same time, method II involved adding a different concentration of HAuCl4 solution to a boiling Na3Cit solution.
In this work, the effectiveness of various developed experiments was compared with the advantages of the Beer-Lambert law since it was necessary to develop cost-benefit methods for synthesizing NPs and appropriate efficiency. Due to the Beer-Lambert equation, the final concentration of synthesized AuNPs correlated directly with surface plasmon resonance (SPR) absorbance at the maximum wavelength. High absorbance was therefore deemed more efficient for the synthesis of AuNPs.
The produced NPs’ UV absorption spectra primarily showed a single absorption peak in the visible region between 510 and 570 nanometers, which was attributed to the spherical AuNPs’ distinctive SPR absorption. Contrarily, non-spherical AuNPs’ UV absorption spectra revealed two SPR bands. Similar outcomes were also reported for spherical AuNPs synthesis using sodium citrate, where maximum SPR peaks were discovered at 517 nanometers for the ultimate particle size of five to 10 nanometers.
Similarly, in both techniques, I and II, the smallest size and most significant SPR absorption were noted at 95-degree Celsius. Although increasing temperatures directly impacted NP size, the maximum SPR absorption was primarily unaffected by the initial temperature, suggesting that the synthesis was efficient. Temperature variations showed no impact on SPR.
The findings also demonstrated that by enhancing the reaction conditions and maintaining the initial pH at a value of approximately 5.5 and a high Na3Cit/HAuCl4 ratio, the uniformity, reproducibility, and size distribution of the final synthesized NPs were accomplished.
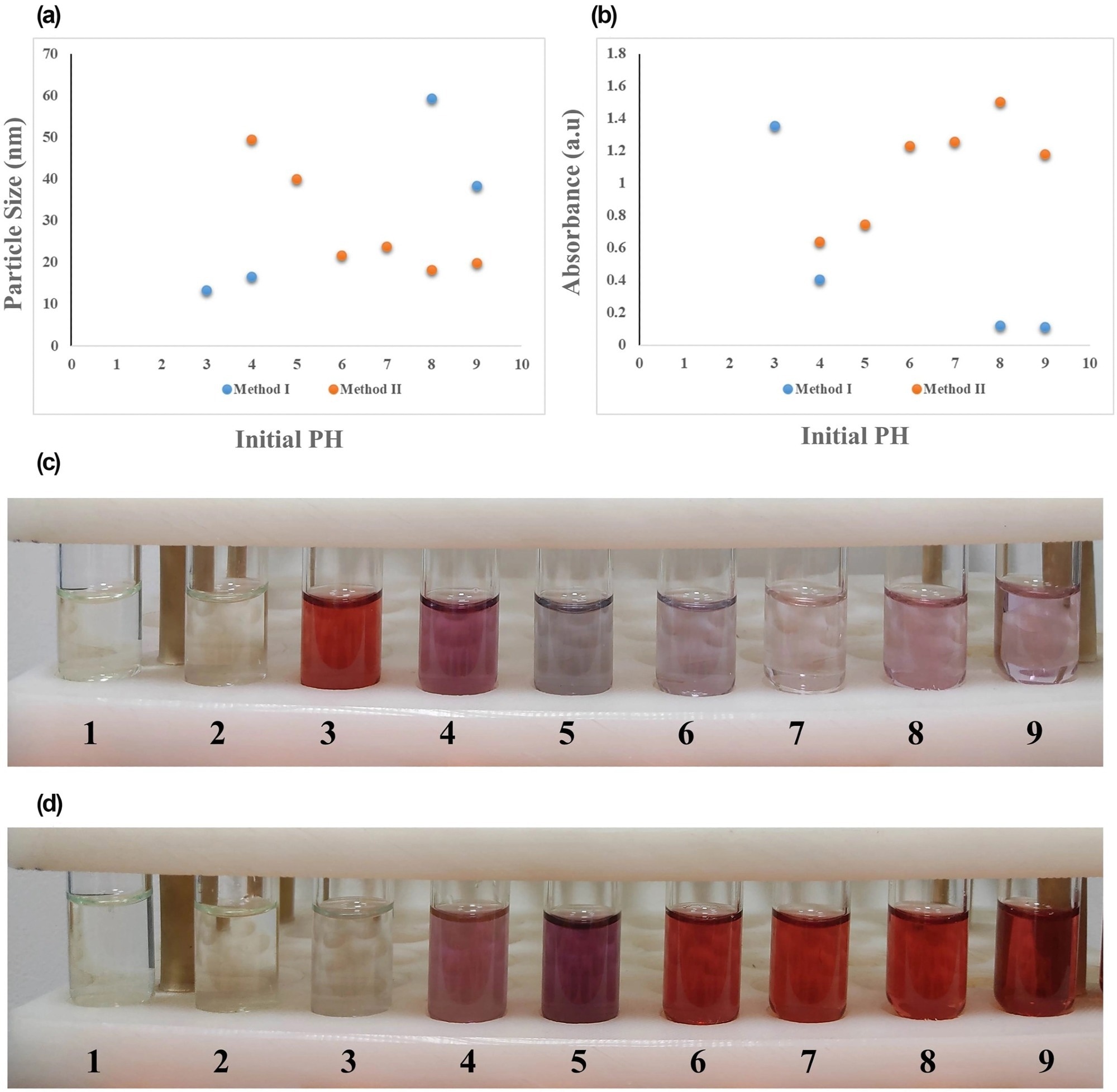
Figure 3. Effect of initial reaction pH values of 1–9 on (a) final particle size and (b) maximum SPR absorption of gold nanoparticles (AuNPs) synthesised by two methods, along with the laboratory images of NPs obtained by (c) method I and (d) method II. SPR, surface plasmon resonance. © Bahmanyar, Z., et al. (2022).
Significance of the Study
The present study conducted several experiments to optimize the synthesis of AuNPs with the final desired efficiency and size distribution by considering various reaction circumstances.
This study showed that, in both the reagent addition orders, primarily in method I, a higher initial reaction temperature and a higher Na3Cit/HAuCl4 molar ratio produced smaller particle sizes with greater synthesis efficacy. The findings also demonstrated the importance of the order of reagent addition and the pH levels on final particle properties.
In conclusion, a 95-degree Celsius temperature value, a five to 5.7 Na3Cit/HAuCl4 molar ratio, and initial pH values of three and seven to eight for methods I and II, respectively, were shown to be the ideal synthesis conditions for producing NPs with reduced final particle sizes and higher synthesis efficacy. The enhanced NPs also displayed crystalline structures, comprising single crystals in the cubic phase and crystallite-sized particles.
Reference
Bahmanyar, Z., et al. (2022). Effect of different physical factors on the synthesis of spherical gold nanoparticles towards cost-effective biomedical applications. IET Nanobiotechnology. https://ietresearch.onlinelibrary.wiley.com/doi/10.1049/nbt2.12100.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.