Self-Assembly of Ligand-Stabilized Nanoparticles
Researchers have shown a great interest in the self-assembly behavior of ligand-stabilized nanoparticles (NPs) over the last few decades. This is attributed to their extraordinary capacity to organize themselves into highly structured structures via simple evaporation techniques.
NP superstructures have already shown exceptional mechanical, photonic, and electrical capabilities, making them suitable for developing novel devices and materials. Iron oxide nanoparticles, in particular, have been extensively researched for their potential applications in a variety of sectors, including sustainable catalysis, healthcare, and nanotechnology.
The shape and faceting of the NP core play a significant role in determining the resulting superlattice structure, with different crystal superstructures reported for truncated nanocubes, ranging from face-centered cubic (FCC), rhombohedral (RH), body-centered tetragonal (BCT), to simple cubic (SC) structures.
The balance between interparticle forces, such as face-face interactions and ligand-ligand interactions, changes with the evolution of NP morphology from perfectly cubic to truncated to spherical, resulting in different structures of the NP superlattices.
Challenges of Self-Assembly of NP Superlattices
To completely comprehend the self-assembly mechanism of superlattices, it is necessary to study the NP core and the ligand shell, the second component portion of the building blocks.
The ligands surrounding the NPs are critical in establishing whether the core shape affects super crystal formation. This is because the solvation and form of the ligand shell may impact the face-face contact of faceted NP cores.
However, two main obstacles must be addressed to fully take advantage of the self-assembly process. The first obstacle is controlling the self-assembly process to produce a fully homogeneous material with few defects.
The second obstacle is developing and improving fabrication pathways that can be scaled up to satisfy industrial demands. This is especially difficult for quick preparation processes like spin-coating because the self-assembly period is relatively brief compared to bulk substance self-assembly by evaporation of the solvent over several weeks.
Highlights of the Current Study
In this study, the authors employed a spin-coating technique to prepare monolayer-controlled nanoparticle films. Adjusting the solubility of the compounds in the solvent allowed the films to be produced.
Based on the solubility of the ligands, the particles were "frozen" in squares or hexagon crystallites in a controlled manner. The crystal structure generated in various solvents was examined to see whether the well-solvated ligand shell covers the fundamental faceting of the NP core, which is not the case in general.
In a "good" solvent, the ligands were virtually entirely spread out, causing the ligand shells to replicate the faceting of the NP core and create a superlattice. The building pieces were cylindrical and self-assembled into hexagonal heterostructures in a "bad" solvent.
The researchers were able to alter the number of implanted NP layers as well as the overall NP coverage of the sample. The nanoparticle layers were discovered to develop layer by layer, revealing structural patterns familiar to atomistic growth.
“This method of construction is typical of how nature produces hard coatings, such as mother-of-pearl or dental enamel,” says Gerold Schneider from the Hamburg University of Technology, a co-author of the study. “Our method now means we are able to create coatings with specific desired properties.”
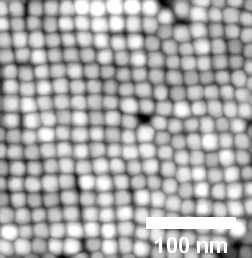
When the solvent chloroform is used, the magnetite nanoparticles arrange themselves into a cubic layer. Image: DESY, Arno Jeromin
Future Prospects
The study demonstrates that spin-coating is an efficient method for fabricating 2D super crystalline lattices of cubic iron oxide nanoparticles with oleyl phosphate ligands onto flat YSZ substrates in minutes.
By varying the duration of the spin-coating process, the researchers were able to control the self-assembly process and the number of deposited monolayers. The study also revealed that the solvent used for the dispersion of the nanoparticles determines the type of 2D lattice that is formed, with toluene resulting in hexagonal layers and chloroform resulting in square layers.
The difference in the Hansen solubility of the ligands in the solvent is suggested to be responsible for the variation in the super crystal building block size and morphology during the self-assembly process.
The spin-coating technique described in this study opens opportunities for creating nanoparticle-functionalized surfaces for multiple applications. Additionally, the prepared 2D layers can serve as templates for the controlled growth of nanoparticle-based 3D bulk materials.
Future research can explore the application of this technique to different nanoparticles, solvents, and substrates to determine the range of materials and applications that can be explored.
Reference
Beck, E. E. et al. (2023). Solvent controlled 2D structures of bottom-up fabricated nanoparticle superlattices. Nanoscale. Available at: https://doi.org/10.1039/D2NR03043H
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.