Jan 22 2009
Each cell in an organism possesses its own protein factories known as ribosomes. Every second, these enzyme complexes produce new proteins with messenger molecules (mRNA) from the cell nucleus as blueprints. In order to generate as many proteins as possible at the same time, several ribosomes cluster together to form an "industrial complex" - the polysome - and read simultaneously the same messenger molecule.
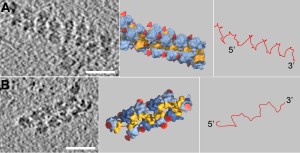 The three dimensional structure of the polysome: Cryoelectron tomographic picture of two polysomes (left), schematic diagram of their structure (middle) and the messenger molecule (mRNA) pathway within the polysome (right). The small ribosomal subunits (yellow) are oriented towards the inside of the polysome, the large subunits (blue) and the nascent protein chains (indicated by red cones) face the cytosol. If the ribosomes are continuously arranged in a "top-to-top" orientation (Fig. A, middle), the result is a pseudo-helical structure of the polysome. If the ribosomes are arranged alternating in "top-to-top" and in "top-to-bottom" orientation (Fig. B, middle), the result is a staggered structure. In both cases the mRNA traverses the shortest possible path from one ribosome to its next neighbor (Fig A and B, right).
The three dimensional structure of the polysome: Cryoelectron tomographic picture of two polysomes (left), schematic diagram of their structure (middle) and the messenger molecule (mRNA) pathway within the polysome (right). The small ribosomal subunits (yellow) are oriented towards the inside of the polysome, the large subunits (blue) and the nascent protein chains (indicated by red cones) face the cytosol. If the ribosomes are continuously arranged in a "top-to-top" orientation (Fig. A, middle), the result is a pseudo-helical structure of the polysome. If the ribosomes are arranged alternating in "top-to-top" and in "top-to-bottom" orientation (Fig. B, middle), the result is a staggered structure. In both cases the mRNA traverses the shortest possible path from one ribosome to its next neighbor (Fig A and B, right).
Scientists at the Max-Planck-Institute of Biochemistry have now, for the first time, been able to reveal the three-dimensional structure of these complexes (Cell 23.1.2009).
In a polysome, the ribosomes are densely packed and exhibit preferred orientations: The small ribosomal subunits are orientated towards the inside of the polysome and the ribosomes are arranged either in a staggered or in a pseudo-helical structure (see figure). This arrangement ensures that the distance between nascent protein chains is maximized, thereby reducing the probability of intermolecular interactions that would give rise to aggregation and limit productive folding. Until now, the belief has been that specialised proteins, the so-called chaperones, would prevent protein misfolding.
Against the background of the new findings, their function appears in a new light: “It appears possible that the main function of chaperones that interact with nascent polypeptide chains is not to suppress chain aggregation within polysomes, but rather to reduce intra-chain misfolding as well as aggregation between different polysomes in the crowded cellular environment”, explains Ulrich Hartl, head of the “Cellular Biochemistry” department, who lead the project in cooperation with Wolfgang Baumeister, head of the “Molecular Structural Biology” department. Moreover, the spatial structure of the polysome enables the ribosomes to process the messenger molecule in the protected area within the polysome and to pass it on without detours. Thus, the architecture of the cellular protein factories facilitates an optimized work flow and increases the efficiency of protein folding.