Mar 1 2018
A method that enables “seeing” beyond the diffraction of light is super-resolution microscopy, which provides unrivaled views through cells and their intrinsic structures and organelles. Recently, the method has gained increasing attention, specifically because the researchers who devised it won, in 2014, the Nobel Prize in Chemistry.
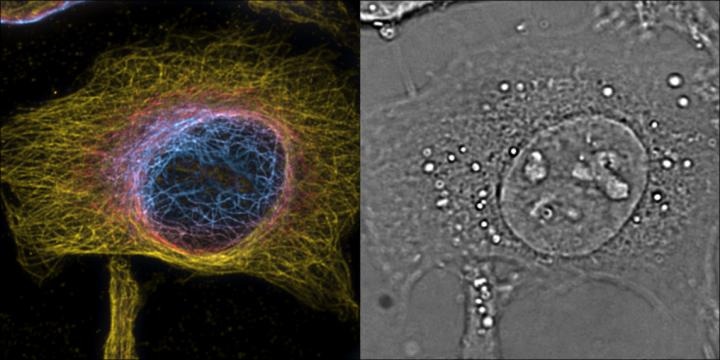 HeLa cells maximum intensity projection of 3D 2nd order bSOFI of labeled microtubules, color encodes z-position with one slice of the complementing 3D phase image providing cellular context. (Image credit: T. Lasser/EPFL)
HeLa cells maximum intensity projection of 3D 2nd order bSOFI of labeled microtubules, color encodes z-position with one slice of the complementing 3D phase image providing cellular context. (Image credit: T. Lasser/EPFL)
However, there is a huge drawback that restricts the application of super-resolution microscopy - it provides only spatial resolution. Although this is adequate for static samples such as fixed cells or solid materials, in the case of biology, matters are highly complicated. Living cells are highly dynamic and are based on a complicated set of biological processes that take place over sub-second timescales, getting constantly modified. Therefore, for visualizing and understanding the behavior of healthy and diseased living cells, a high time, or “temporal,” resolution is also needed.
A research group headed by Professor Theo Lasser, Head of the Laboratory of Biomedical Optics (LOB) at EPFL, has now taken longer steps to overcome the challenge by devising a method with the potential to carry out both fast 3D phase imaging and 3D super-resolution microscopy in a single instrument. Phase imaging is a method in which the alterations in the phase of light brought about by cells and their organelles are converted into refractive index maps of the cells themselves.
The distinctive platform, termed as a “4D microscope,” integrates the high time-resolution and sensitivity of phase imaging with the high spatial resolution and specificity of fluorescence microscopy. The scientists created an innovative algorithm with the ability to recover the phase information from a pile of bright-field images captured by a classical microscope.
With this algorithm, we present a new way to achieve 3D quantitative phase microscopy using a conventional bright-field microscope. This allows direct visualization and analysis of subcellular structures in living cells without labeling.
Adrien Descloux, Lead Author
To accomplish fast 3D imaging, the researchers developed a tailor-made image-splitting prism that enables concurrent recording of a stack of eight z-displaced images. This indicates that the microscope can carry out high-speed 3D phase imaging over a volume with dimensions of 2.5 μm ´ 50 μm ´ 50 μm. The speed of the microscope is fundamentally restricted by the speed of its camera. For this illustration, the researchers were able to image intracellular dynamics at nearly 200 Hz.
“With the prism as an add-on, you can turn a classical microscope into an ultra-fast 3D imager,” stated Kristin Grussmayer, another lead authors of the study.
The prism can also be used for 3D fluorescence imaging, which was investigated by the researchers by adopting super-resolution optical fluctuation imaging (SOFI). This technique takes advantage of the blinking of fluorescent dyes to enhance 3D resolution by carrying out correlation analysis of the signal. By adopting this, the team carried out 3D super-resolution imaging of stained structures in the cells, and integrated it with 3D label-free phase imaging. The two methods complemented each other quite well, exhibiting intriguing images of the intrinsic structure, cytoskeleton, and organelles in living cells over distinctive time points.
“We are thrilled by these results and the possibilities offered by this technique,” stated Professor Hilal Lashuel, whose lab at EPFL collaborated with Professor Lasser’s lab in adopting the innovative method to investigate the mechanisms by which protein aggregation plays a vital role in the development and advancement of neurodegenerative diseases, for example, Alzheimer’s and Parkinson’s. “The technical advances enabled high-resolution visualization of the formation of pathological alpha-synuclein aggregates in hippocampal neurons.”
The researchers have termed the innovative microscopy platform as PRISM, which stands for Phase Retrieval Instrument with Super-resolution Microscopy.
We offer PRISM as a new microscopy tool and anticipate that it will be rapidly used in the life science community to expand the scope for 3D high-speed imaging for biological investigations. We hope that it will become a regular workhorse for neuroscience and biology.
Theo Lasser
This study was funded by the European Union (Horizon 2020, Marie Skłodowska-Curie Grant Agreement and AD-gut European consortium) and the Swiss National Science Foundation (SNSF).
Reference
A. Descloux, K. S. Grußmayer, E. Bostan, T. Lukes, A. Bouwens, A. Sharipov, S. Geissbuehler, A-L. Mahul-Mellier, H. A. Lashuel, M. Leutenegger, T. Lasser. Combined Multi-Plane Phase Retrieval and Super-Resolution Optical Fluctuation Imaging for 4D Cell Microscopy. Nature Photonics 12, 165-172 (26 February 2018). DOI: 10.1038/s41566-018-0109-4