Jul 29 2010
A normally benign protein found in the human body appears to be able – when paired with nanoparticles – to zero in on and kill certain cancer cells, without having to also load those particles with chemotherapy drugs.
The finding could lead to a new strategy for targeted cancer therapies, according to the University of North Carolina at Chapel Hill scientists who made the discovery.
However, they also cautioned that the result raises concerns about unanticipated “off-target” effects when designing nano-delivery agents.
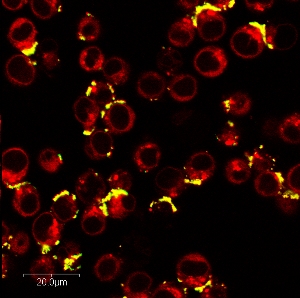 Transferrin-carrying nanoparticles that have targeted and permeated Ramos cancer cells. Areas of yellow represent the intracellular compartments of the cells where the nanoparticles reside. Areas of red represent intracellular compartments without nanoparticles.
Transferrin-carrying nanoparticles that have targeted and permeated Ramos cancer cells. Areas of yellow represent the intracellular compartments of the cells where the nanoparticles reside. Areas of red represent intracellular compartments without nanoparticles.
Transferrin, the fourth most abundant protein in human blood, has been used as a tumor-targeting agent for delivering cancer drugs for almost two decades. The protein’s receptor is over-expressed on the surface of many rapidly growing cancers cells, so treatments combined with transferrin ligands are able to seek out and bind to them. Nanoparticles infused with transferrin have long been regarded as safe and nontoxic.
Now, UNC researchers have shown that, surprisingly, attaching transferrin to a nanoparticle surface can effectively and selectively target and kill B-cell lymphoma cells, found in an aggressive form of non-Hodgkin’s lymphoma. It had been thought that nanoparticles would also need to carry toxic chemotherapy agents to have such an effect.
The discovery was made by a team of researchers led by Joseph DeSimone, Ph.D., Chancellor’s Eminent Professor of Chemistry in UNC’s College of Arts and Sciences and William R. Kenan Jr. Distinguished Professor of Chemical Engineering at North Carolina State University, along with Jin Wang, Ph.D., and Shaomin Tian, Ph.D., in DeSimone’s lab. Their findings appear in this week’s online issue of the Journal of the American Chemical Society.
The scientists say the result is an interesting development in the field of nanomedicine, which researchers hope will eventually provide widely accepted alternatives – or replacements – to chemo and radiation treatment. Those therapies, while considered the most effective methods currently available for tackling cancer, also often damage healthy tissues and organs as a side effect.
Using PRINT (Particle Replication in Non-wetting Templates) technology — a technique invented in DeSimone’s lab that allows scientists to produce nanoparticles with well-defined size and shape — the UNC researchers produced biocompatible nanoparticles bonded with human transferrin, and demonstrated that the particles can safely and accurately recognize a broad spectrum of cancers. As well as B-cell lymphoma cells, the particles also effectively targeted non-small cell lung, ovarian, liver and prostate cancer cells.
Generally, the nanoparticles were non-toxic to such cells and should therefore be able to be loaded with standard chemotherapy agents and used to hone in on those cancers.
However, for Ramos cells, an aggressive form of B-cell lymphoma, the transferrin-bonded PRINT nanoparticles not only recognized them but also induced cell death. Meanwhile, free transferrin – which was incubated with Ramos cells but not bound to any nanoparticles – did not kill any Ramos cells, even at high concentrations.
Researchers are carrying out further studies to determine how and why the transferrin-carrying nanoparticles proved toxic to the Ramos cells but not the other tumor types.
“Although this is potentially exciting for the development of entirely new strategies for treating certain types of lymphomas with potentially lower side effects, this study also raises concerns for unanticipated off-target effects when one is designing targeted chemotherapy agents for other types of cancers,” said DeSimone. DeSimone is also a member of UNC’s Lineberger Comprehensive Cancer Center and the co-principal investigator for the Carolina Center for Cancer Nanotechnology Excellence. He was also recently appointed as an adjunct member at New York’s Memorial Sloan-Kettering Cancer Center.
Other UNC coauthors were Robby A. Petros, Ph.D., and Mary E. Napier, Ph.D., from the chemistry department and the Carolina Center for Cancer Nanotechnology Excellence.
The study, “The Complex Role of Multivalency in Nanoparticles Targeting the Transferrin Receptor for Cancer Therapies,” was funded by the National Science Foundation; the National Institutes for Health; the Carolina Center for Cancer Nanotechnology Excellence; the William R. Kenan Professorship; Liquidia Technologies; and the North Carolina University Cancer Research Fund, established by the N.C General Assembly to help prevent and treat cancer across the state.
Source: http://www.unc.edu/