May 27 2019
An international team of scientists has recently enhanced the ability of graphene to catalyze the “hydrogen evolution reaction,” which produces hydrogen upon passing an electric current via water during electrolysis.
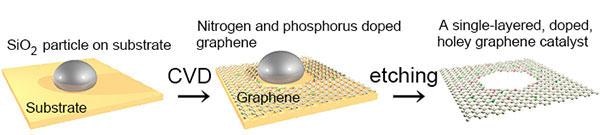 Carbon atoms were deposited on a substrate using chemical vapor deposition. Silicon oxide nanoparticles on the substrate ensured the formation of holes. Nitrogen and phosphorus atoms were added. Ultimately a single-layered, doped, holey graphene catalyst was formed. (Image credit: Kumatani)
Carbon atoms were deposited on a substrate using chemical vapor deposition. Silicon oxide nanoparticles on the substrate ensured the formation of holes. Nitrogen and phosphorus atoms were added. Ultimately a single-layered, doped, holey graphene catalyst was formed. (Image credit: Kumatani)
The researchers engineered a mathematically-predicted graphene electrocatalyst, and validated its performance with the help of computational modeling and high-resolution electrochemical microscopy. The results of the study have been reported in the journal, Advanced Science.
Yoshikazu Ito of Tsukuba University, Akichika Kumatani from Advanced Institute for Materials Research (AIMR) at Tohoku University, Tatsuhiko Ohto of Osaka University, and colleagues in Germany and Japan discovered that when phosphorus and nitrogen “dopants” are added around the well-characterized edges of graphene holes, its potential to electrocatalyze the hydrogen evolution reaction is considerably improved.
When compared to metal-based catalysts, graphene-based ones provide an advantage in that they are controllable and stable, rendering them appropriate for use in water electrolysis, energy storage and conversion devices, and fuel cells. Making numerous concurrent changes to their structures can enhance the properties of graphene-based catalysts. However, researchers need to be able to “see” these variations at the nanoscale so as to figure out the way they operate together to encourage catalysis.
Along with his colleagues, Kumatani applied the newly developed scanning electrochemical cell microscopy, or SECCM, for direct and sub-microscale visualization of the electrochemical reactions that take place upon passing an electric current through water during electrolysis. In addition, it also enabled the researchers to examine how structural variations in graphene electrocatalysts have an impact on their electrochemical activities. An observation like this cannot be achieved using traditional methods.
The researchers developed a novel electrocatalyst fabricated from a graphene sheet full of scientifically predicted holes with well-characterized edges. These edges around the graphene holes boost the number of active sites existing for chemical reactions to take place. To dope the graphene sheet, the team added phosphorus and nitrogen atoms around the hole edges. Subsequently, the graphene-based electrocatalyst was used for improving the release of hydrogen at the time of electrolysis.
With the help of the SECCM technique, the researchers discovered that their graphene-based electrocatalyst considerably enhanced the formation of an electric current in response to the energy produced during electrolysis. The computational computations of the team indicate that the addition of phosphorus and nitrogen dopants improves the contrast of negative and positive charges on the atoms enclosing the hole edges, thereby increasing their potential to pass an electric current.
It was observed that holey graphene electrocatalysts doped with phosphorus and nitrogen functioned better when compared to the ones doped with just one of the two chemical elements.
“These findings pave a path for atomic-level engineering of the edge structure of graphene in graphene-based electrocatalysts through the local visualization of electrochemical activities,” concluded the scientists.