Worldwide, bacterial infections associated with medical implants place a heavy burden on healthcare and lead to great suffering in patients.
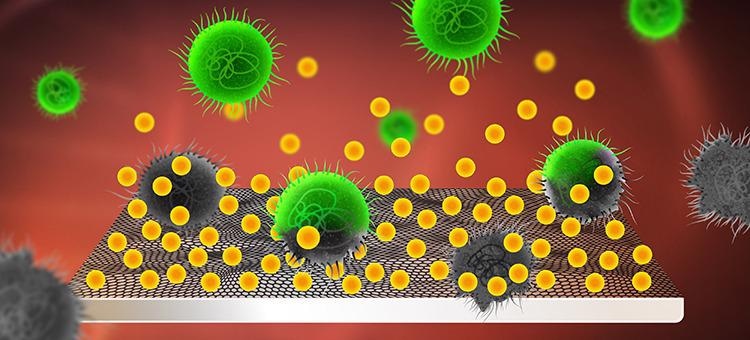 Usnic acid (yellow) is integrated into and released from the implant’s graphene coating. The usnic acid kills the bacteria (green) and prevents them from forming infectious biofilms on the surface. Image Credit: Yen Strandqvist/Chalmers.
Usnic acid (yellow) is integrated into and released from the implant’s graphene coating. The usnic acid kills the bacteria (green) and prevents them from forming infectious biofilms on the surface. Image Credit: Yen Strandqvist/Chalmers.
Scientists at Chalmers University of Technology (Chalmers) have now developed a new method for preventing such infections. It works by covering a graphene-based material using bactericidal molecules.
Through our research, we have succeeded in binding water-insoluble antibacterial molecules to the graphene, and having the molecules release in a controlled, continuous manner from the material.
Santosh Pandit, Study First Author and Researcher, Department of Biology and Biological Engineering, Chalmers University of Technology
“This is an essential requirement for the method to work. The way in which we bind the active molecules to the graphene is also very simple, and could be easily integrated into industrial processes,” added Pandit.
The study was recently reported in the Scientific Reports journal.
Some bacteria have the ability to form impenetrable surface layers, or so-called “biofilms,” on surgical implants, like dental and other orthopedic implants, and pose a serious issue for healthcare across the globe.
Biofilms are regarded as highly resistant compared to other bacteria, and hence the infections seem to be often difficult to treat, resulting in huge suffering for patients. In the most serious cases, implant removal or replacement is needed. Besides the effects on patients, this incurs high costs for healthcare providers.
An extensive range of water-insoluble or hydrophobic drugs and molecules can be utilized for their antibacterial properties. Still, to be used within the body, they must be fixed to a material, which can be hard and time-consuming to manufacture.
Graphene offers great potential here for interaction with hydrophobic molecules or drugs, and when we created our new material, we made use of these properties. The process of binding the antibacterial molecules takes place with the help of ultrasound.
Santosh Pandit, Study First Author and Researcher, Department of Biology and Biological Engineering, Chalmers University of Technology
The graphene material was covered using usnic acid, derived from lichens, for instance, fruticose lichen. Usnic acid exhibits good bactericidal properties, which was proven by a previous study.
The function of usnic acid is to prevent bacteria from developing nucleic acids, which especially inhibits the RNA synthesis, and hence blocking cells’ protein production. Usnic acid was subjected to test for its resistance to the pathogenic bacteria Staphylococcus epidermidis and Staphylococcus aureus. These two bacteria are known to be the common culprits for biofilm formation on medical implants.
The newly developed material exhibited several useful properties. Besides obtaining successful outcomes for combining usnic acid into the graphene material’s surface, the researchers also noted that usnic acid molecules were discharged in a constant and regulated way. This helped prevent the formation of biofilms on the surface.
Even more importantly, our results show that the method for binding the hydrophobic molecules to graphene is simple. It paves the way for more effective antibacterial protection of biomedical products in the future. We are now planning trials where we will explore binding other hydrophobic molecules and drugs with even greater potential to treat or prevent various clinical infections.
Santosh Pandit, Study First Author and Researcher, Department of Biology and Biological Engineering, Chalmers University of Technology
Journal Reference:
Pandit, S., et al. (2021) Sustained release of usnic acid from graphene coatings ensures long-term antibiofilm protection. Scientific Reports. doi.org/10.1038/s41598-021-89452-5.