Once thought of as a game-changer in predicting cancer drug performance, organoids have delivered mixed results outside the lab. Now, scientists are pairing them with precision-engineered nanoparticles in an effort to redeem the promise: to better forecast which treatments might succeed in patients, and to understand why so many still fail.
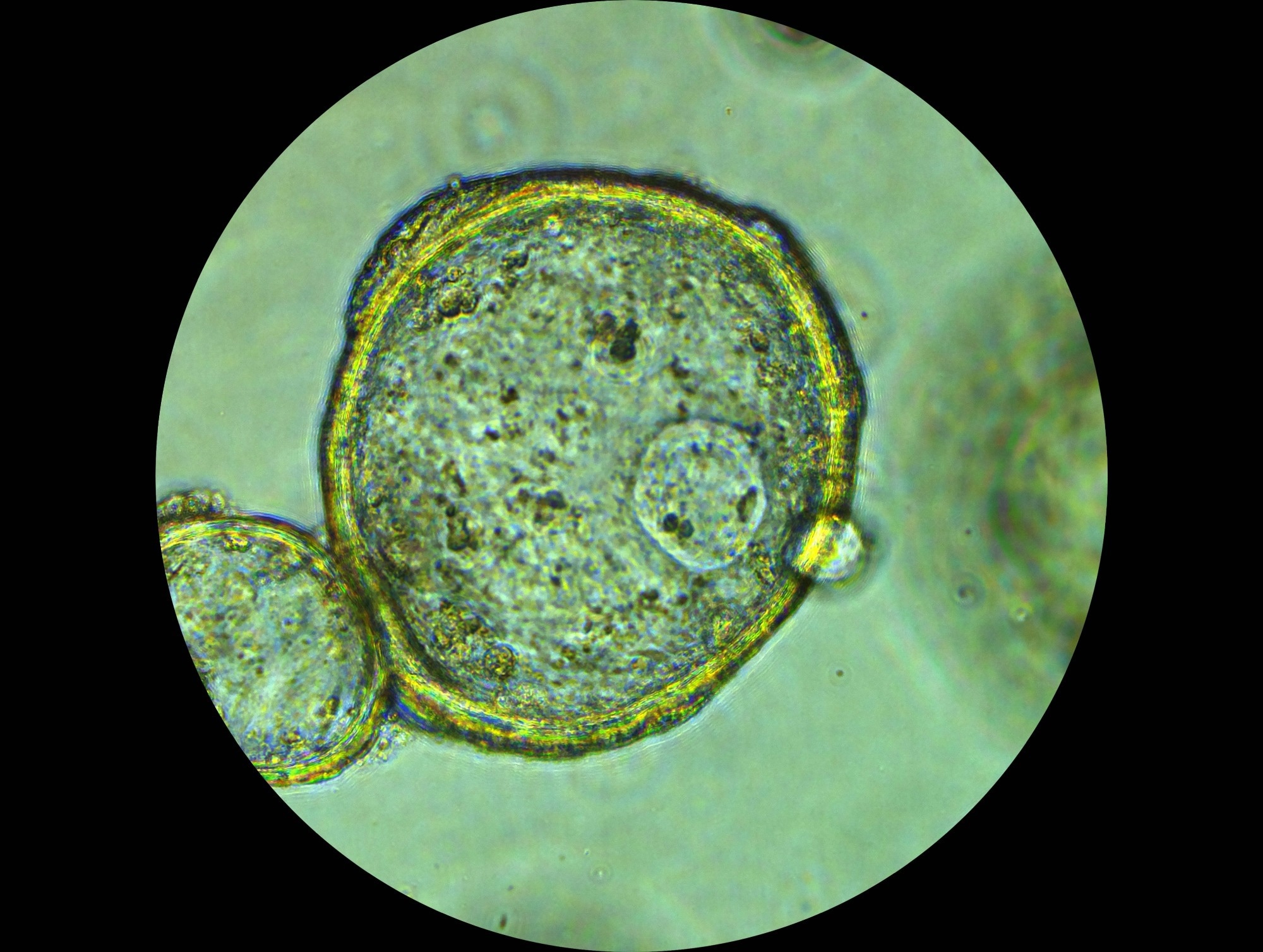
Image Credit: Nthielen/Shutterstock.com
Organoids, tiny replicas of human organs, are grown from stem cells or tumor cells and develop into three-dimensional structures containing many of the same cell types as the real organ or tissue. They can self-renew and self-organize, which makes them useful for studying disease progression, testing drugs, and even exploring personalised medicine.1,2
But there are limits. While organoids mimic much of a tumor’s biology, they rarely match its full complexity. Most lack blood vessels, immune cells, and the intricate systemic interactions that influence how a treatment works in real-life treatments.
Nanoparticles Need Better Testing Grounds
Nanoparticles can carry cancer drugs directly to tumors, release them slowly, and limit damage to healthy cells. They can transport everything from small molecules to proteins, nucleic acids, and chemotherapy agents.3
On paper, and often in early lab studies, they have looked highly effective. But many nanomedicines falter when they reach clinical trials. One major reason is the tumor microenvironment: a dense, protective network of cells and extracellular material that limits drug penetration.4
Traditional flat, two-dimensional cell cultures do little to capture this complexity. Organoids, though not perfect, offer a more realistic model for testing how nanoparticles behave with their three-dimensional architecture.
Download your PDF copy now!
Evidence From Different Cancers
Pancreatic ductal adenocarcinoma organoids can replicate the dense, treatment-resistant environment of pancreatic tumors. Studies of magnetic nanoparticles for hyperthermia therapy found they tended to stick to cell surfaces rather than enter cells, suggesting limits to their delivery potential. Other work showed that tumor-penetrating nanocomplexes could deliver genetic material (siRNA) effectively in both human and mouse pancreatic ductal adenocarcinoma organoids.5,6
Breast cancer organoids can be used to model a range of subtypes, including giant papillary breast cancer. In one experiment, organoids from oestrogen receptor-positive tumors were treated with PPFA-cRGD nanoparticles carrying two targeted drugs. The nanoparticles caused more cell death than chemotherapy alone. Another study tested sulfur-doped carbon nanodots and achieved encouraging results in patient-derived organoids.6
Colorectal cancer organoids maintain the glandular structure and genetic diversity of the disease, features often missing from earlier organoid models. Researchers have used them to test multifunctional nanoparticles, including a curcumin-based system that showed therapeutic potential against lab-grown colorectal cancer tissue.6
Glioblastoma is a deadly brain cancer with high resistance and relapse rates. Brain organoids, modelling tumors such as glioblastoma and meningioma, have helped track how nanoparticles cross the blood-brain barrier and release drugs inside tumors. Gold nanoparticles carrying both a chemotherapy drug and a fluorescent tag allowed researchers to monitor delivery and drug release in real time.6,7
The Persistent Gaps
Despite the advantages, organoids are not a perfect stand-in for patients. Their lack of vascular and immune systems affects how drugs behave. They also cannot fully replicate systemic processes like liver metabolism or kidney clearance, which are critical to understanding dosage and side effects.
This means that while organoid-nanoparticle systems can reveal much more than flat cultures, they are still not able to guarantee that a promising result in the lab will succeed in real life.7
The Future of Drug Development: How Organoids Are Leading the Charge
What Comes Next
Researchers are working to improve organoid complexity by adding vascular structures and immune components, creating models that behave more like actual tumors. Large biobanks of patient-derived organoids are also being built, allowing high-throughput testing of nanoparticle-based drugs.
If these efforts succeed, organoids could eventually become an important tool in cancer drug development, giving trials a firmer foundation and a greater chance of success in treating cancers in patients.
References
- Yang S, et al. Organoids: The current status and biomedical applications. MedComm (2020). 2023;4(3):e274. doi: 10.1002/mco2.274.
- Ryu JO, et al. Applications and research trends in organoid-based infectious disease models. Sci Rep. 2025;15(1):25185. doi: 10.1038/s41598-025-07816-7..
- Yao Y, et al. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front Mol Biosci. 2020;7:193. doi: 10.3389/fmolb.2020.00193.
- Abdullah KM, et al. Nanomedicine in Cancer Therapeutics: Current Perspectives from Bench to Bedside. Mol Cancer. 2025;24(1):169. doi: 10.1186/s12943-025-02368-w.
- Lv J, et al. Construction of tumor organoids and their application to cancer research and therapy. Theranostics. 2024;14(3):1101-1125. doi: 10.7150/thno.91362.
- Chen L, et al. Harnessing Organoid Platforms for Nanoparticle Drug Development. Drug Des Devel Ther. 2025;19:6125-6143. doi: 10.2147/DDDT.S530999.
- Fang Z, et al. The role of organoids in cancer research. Exp Hematol Oncol. 2023 Aug 3;12(1):69. doi: 10.1186/s40164-023-00433-y.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.