MXenes, a promising class of 2D materials with diverse applications, have faced manufacturing challenges due to expensive, complex, and wasteful production methods. A new technique offers a more efficient method by synthesizing MXenes atom-by-atom from the bottom up.
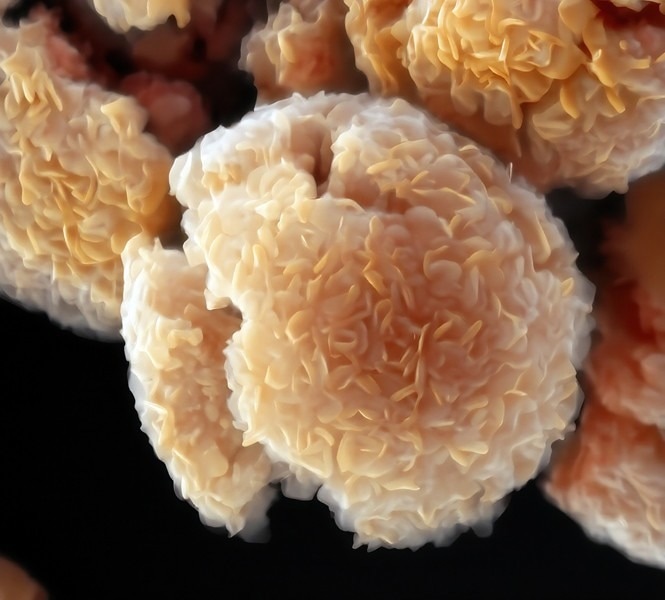 A colorized scanning electron microscopy (SEM) image of a two-dimensional material called a MXene. This particular MXene, a combination of niobium, carbon, and chlorine, was synthesized using a new "bottom-up" method pioneered by researchers from the University of Chicago, University of Illinois Chicago, and Vanderbilt University through the NSF Center for Chemical Innovation on MXenes Synthesis, Tunability and Reactivity (M-STAR). Image Credit: UChicago Talapin Lab
A colorized scanning electron microscopy (SEM) image of a two-dimensional material called a MXene. This particular MXene, a combination of niobium, carbon, and chlorine, was synthesized using a new "bottom-up" method pioneered by researchers from the University of Chicago, University of Illinois Chicago, and Vanderbilt University through the NSF Center for Chemical Innovation on MXenes Synthesis, Tunability and Reactivity (M-STAR). Image Credit: UChicago Talapin Lab
Developed by researchers at the University of Chicago, the University of Illinois at Chicago, and Vanderbilt University, the study was published in the journal Nature Synthesis.
MXenes (pronounced as "Maxine") represent a category of two-dimensional materials that were first discovered merely 14 years ago. MXenes possess extraordinary potential for applications in energy storage, catalysis, ultra-strong lightweight composites, and numerous other uses, including electromagnetic shielding and conductive inks.
However, the production of MXenes has proven to be costly, challenging, and overly simplistic.
MXenes have been made by a very elaborate, multi-step process that involved days of high-temperature work, followed by using dangerous chemicals like hydrofluoric acid, and created a lot of waste. That may have been okay for early-stage research and lab exploration, but became a big roadblock for taking the next step to large-scale applications.
Dmitri Talapin, Professor, School of Molecular Engineering and Department of Chemistry, University of Chicago Pritzker
The study, facilitated by the NSF Center for Chemical Innovation on MXenes Synthesis, Tunability and Reactivity (M-STAR), and employed chemical vapor deposition to produce MXenes that are "at least two orders of magnitude" more affordable than those synthesized through conventional techniques, according to Talapin, the UChicago Ernest DeWitt Burton Distinguished Service Professor.
“What’s exciting about this paper is it’s a new way of doing chemical synthesis, using a new set of organic precursors, that allows us to achieve these 2D materials more efficiently,” said De-en Jiang, the H. Eugene McBrayer Professor and Study Co-Author, Chemical Engineering, at Vanderbilt University.
The innovative technique for producing this advanced material was influenced by a little-known article authored by a renowned chemist, which was published in 1986.
“We came across a forgotten paper by the great John Corbett at Iowa State University that very few people knew about and that showed the chemistry that we found inspirational for the development of our ideas,” said Talapin.
Corbett's research outlined a technique for synthesizing layered zirconium chloride carbide, which bears structural similarities to another material that was first introduced 25 years later – MXene.
MXenes consist of layers of transition metals that are so thin at the atomic level that they are most accurately characterized as two-dimensional developed by researchers at Drexel University in 2011. Scientists globally are exploring their potential applications in energy storage, industrial catalysts, electromagnetic interference shielding, optoelectronics, and various other innovative fields.
“MXenes are widely explored, particularly for energy-storage applications, because they consist of conductive two-dimensional layers that can host ions between them. They also have tunable surface groups, which can be chemically tailored to control which ions are stored, how favorable that storage is, and how efficiently ions flow into and out of the layers,” said Noah Mason, a Ph.D. Student, Study Co-Author, and NSF Graduate Research Fellow.
It’s like trying to carve a book out of a block of wood. Our new method builds that book the way it should be made – page by page.
Robert Klie, Study Co-Author and Professor, Head, Physics Department, University of Illinois Chicago
Behind the Synthesis
The adaptation of the synthesis methods described in Corbett's paper, transitioning from metals such as zirconium to the titanium present in the most prevalent MXenes, resulted in a publication in Science in 2023. However, numerous challenges remained before the technique could be widely implemented.
“In the 2023 paper, we didn't show a very high yield or purity of the MXenes in our final product. We could not make it higher than 60 weight percent. In this paper, we achieved 90 weight percent. We not only discovered a new reaction, but started to learn about the secret behind the synthesis,” said Di Wang, the first author of both the 2023 and 2025 papers.
Safety and cost were significant concerns, stated Wang, who was a Ph.D. student at UChicago in Talapin’s lab during the research, and is currently a postdoctoral researcher at Princeton University.
For instance, the precursor chemical utilized in that previous study – titanium tetrachloride – is highly reactive, and researcher Wang recalls it etching the plastic pipette syringes while he attempted to use them. The new research employs tetrachloroethylene, a chemical that is both affordable and stable, making it commonly used to extract caffeine from coffee beans for decaffeinated beverages.
Talapin stated that research building upon Corbett's work four decades later demonstrates the significance of pure exploratory research – science conducted for its own sake, allowing future scientists to discover practical applications for the results.
The M-STAR team is well-positioned to help. The consortium lets chemists use traditional inorganic chemistry, nanosynthesis, catalysis, or other novel, interdisciplinary approaches, coupled with computational modeling and simulations, to attack problems from different angles.
De-en Jiang, H. Eugene McBrayer Professor and Study Co-Author, Chemical Engineering, Vanderbilt University
“The M-STAR CCI is pioneering an approach where the chemist is front and center. They’re going to be using MXenes as a platform to drive innovation in chemistry by working together with materials scientists, physicists, and chemical engineers in the team,” said Jiang.
Journal Reference:
Wang, D., et al. (2025) Molecular organohalides as general precursors for direct synthesis of two-dimensional transition metal carbide MXenes. Nature Synthesis. DOI:10.1038/s44160-025-00946-w. https://www.nature.com/articles/s44160-025-00946-w