Using an enhanced form of "chemical microscopy" developed at the National Institute of Standards and Technology (NIST), researchers there have shown that they can peer into the structure of blended polymers, resolving details of the molecular arrangement at sub-micrometer levels.* The capability has important implications for the design of industrially important polymers like the polyethylene blends used to repair aging waterlines.
 Typical BCARS composite image of a polyethylene blend taken at NIST showing circular polarization response. LLD polyethylene shows red in this mode, while the HD polyethylene with deuterium substituted for hydrogen is green. (Credit: NIST)
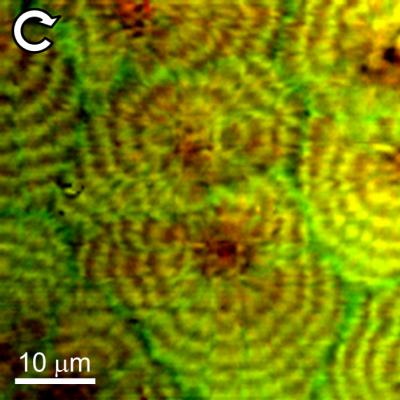
Typical BCARS composite image of a polyethylene blend taken at NIST showing circular polarization response. LLD polyethylene shows red in this mode, while the HD polyethylene with deuterium substituted for hydrogen is green. (Credit: NIST)
Polyethylene is one of the most widely produced and used polymers in the world. It's used in many familiar applications—milk bottles, for instance—but the NIST research is motivated by a more critical application: water pipes. Aging water infrastructure is a significant national issue. The Environmental Protection Agency has reported that in the United States there are over 240,000 water main breaks per year, leaks wasting 1.7 trillion gallons of water per year, and costs to taxpayers of $2.6 billion per year.
Polyethylene pipes are one potential solution. They're relatively inexpensive to make and install, and they have negligible corrosion issues and a predicted service life of up to a century under ideal conditions. Unfortunately, current test standards do not address service life under field conditions, especially for fusion joints in the pipes. This uncertainty has slowed the use of large diameter polyethylene pipe.
The industry standard for polyethylene pipes is a blend of two different forms of the polymer, a medium-weight, high-density polyethylene (HDPE) and a high molecular weight "linear low-density polyethylene" (LLDPE). Combining the two, says NIST materials scientist Young Jong Lee, dramatically improves the toughness, strength and resistance to fracture of the polymer.
The problem for quantitative service-life prediction is understanding exactly why that is. Developing the necessary predictive models has been hindered by knowing just how the HDPE and LLDPE molecules blend together. They are so close chemically that X-ray or electron imaging—the usual go-to techniques for molecular structure—can't readily distinguish them.
The NIST team is using a variation of Raman spectroscopy, which can distinguish different chemical species—and measure how much of each—by analyzing the frequencies associated with the different vibrational modes of each molecule. The exact mix of these frequencies is an extremely discriminating "fingerprint" for any particular molecule without help of fluorescence labeling. Raman spectroscopy using focused laser beams has been used as a chemical microscope, able to detail the structure of complex objects by mapping the chemical composition at each point in a three-dimensional space.
The NIST instrument, called "BCARS" (broadband coherent anti-Stokes Raman scattering) microscopy, uses a pair of lasers to gather Raman data at least 10 times faster than other Raman imaging methods, a critical feature because of the vast amount of data that must be gathered to understand such highly structured blend systems.** The extra trick is to substitute deuterium ("heavy hydrogen") for hydrogen atoms in the HDPE component. The deuterium strongly shifts the Raman spectrum, making it easy to distinguish the two components. By controlling the polarization of the light, the technique provides additional details on the local crystal orientation of molecules in the polymer. The images show, for example, the formation of microscopic spherical regions of partial crystallization with the LLDPE more concentrated towards the center.
"This is a fast, three-dimensional chemical imaging technique that's particularly useful for studying microstructures of polymeric materials," says Lee.